Highly photocatalytic performance of TiO2 nanowires in the conversion of benzaldehydes to benzoic acid
Authors
Sudirman Sudirman , Lalu Dimas Pratama Atmaja , Ari Jamhari Pratama , Emmy Yuanita , Ni Komang Tri Dharmayani , Maria Ulfa , Romel HidayatDOI:
10.29303/aca.v6i2.160Published:
2023-06-04Issue:
Vol. 6 No. 2 (2023)Keywords:
Nanomaterials, Photocatalyst, TiO2 NPs, TiO2 NWs, Benzaldehyde, Benzoic acidArticles
Downloads
How to Cite
Downloads
Metrics
Abstract
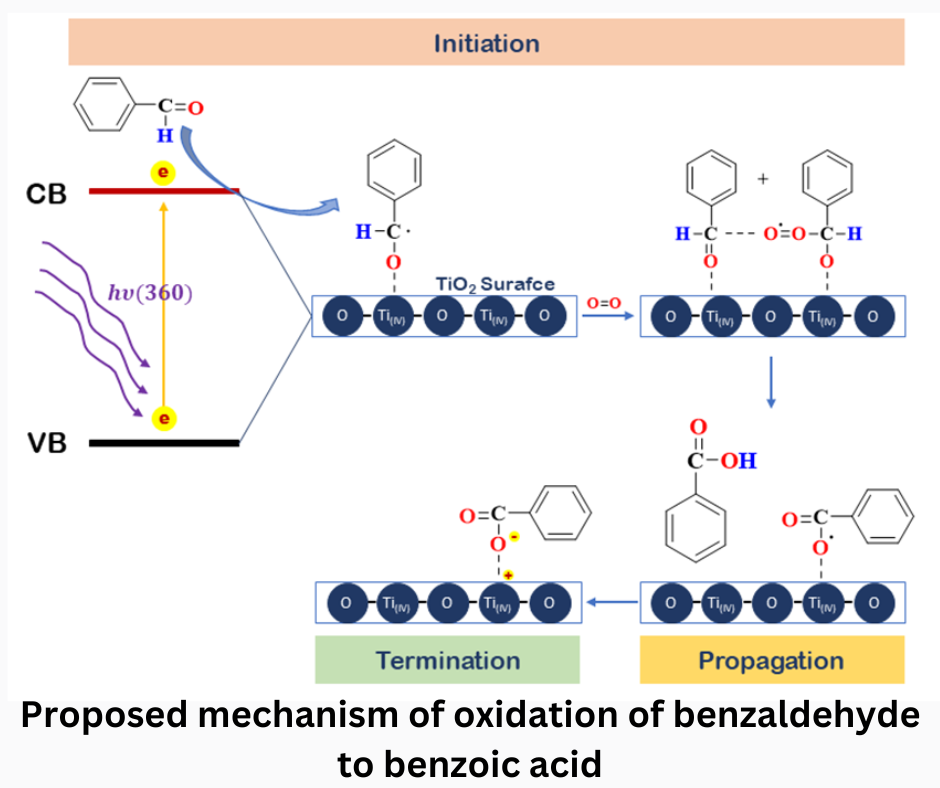
The synthesis of environmentally friendly-based chemicals such as solvent-free continues to be developed. A critical precursor in chemical synthesis is benzoic acid. This research developed a synthesis method by utilizing TiO2 nanomaterials with different morphologies as photocatalysts, namely nanoparticles (NPs) and nanowires (NWs). Titanium (IV) oxide with nanowires morphology was synthesized by hydrothermal method under alkaline conditions. SEM, XRD, and FT-IR images confirmed the morphologies of TiO2 NPs and TiO2 NWs. Photocatalytic performance in converting benzaldehyde to benzoic acid showed a significant difference of up to 38% using TiO2 NPs and 94% using TiO2 NWs.
References
H. Yan, C. Liu, and G. Luo, “Oxidation of benzaldehyde to benzoic acid,” Pet Sci Technol, vol. 23, no. 11–12, pp. 1511–1516, Nov. 2005, doi: 10.1081/LFT-200041057.
D. J. P. Hogg, M. North, R. B. Stokoe, and W. G. Teasdale, “The Oxidation of Benzaldehyde to Benzoic Acid Catalysed by Cycle-[(S)-His-(S)-Phe], and its Implications for the Catalytic Asymmetric Addition of HCN to Aldehydes,” 1993.
X. Deng and C. M. Friend, “Selective oxidation of styrene on an oxygen-covered Au(111),” J Am Chem Soc, vol. 127, no. 49, pp. 17178–17179, Dec. 2005, doi: 10.1021/ja0557031.
B. Mutsuo Kodama et al., “Stability Constants of Metal Ions Complexes,” The Chemical Society, 1967.
Z. Zhu and J. H. Espenson, “Articles Oxidation of Alkynes by Hydrogen Peroxide Catalyzed by Methylrhenium Trioxide,” 1995.
P. Chowdhury, G. Malekshoar, M. B. Ray, J. Zhu, and A. K. Ray, “Sacrificial hydrogen generation from formaldehyde with Pt/TiO2 photocatalyst in solar radiation,” Ind Eng Chem Res, vol. 52, no. 14, pp. 5023–5029, Apr. 2013, doi: 10.1021/ie3029976.
L. X. Zhang, Z. le Hua, X. P. Dong, L. Li, H. R. Chen, and J. L. Shi, “Preparation of highly ordered Fe-SBA-15 by physical-vapor-infiltration and their application to liquid phase selective oxidation of styrene,” J Mol Catal A Chem, vol. 268, no. 1–2, pp. 155–162, May 2007, doi: 10.1016/j.molcata.2006.12.027.
M. Dhiman and S. Singhal, “ScienceDirect Enhanced catalytic properties of rare-earth substituted cobalt ferrites fabricated by sol-gel auto-combustion route,” 2019. [Online]. Available: www.sciencedirect.com
J. Liu, Z. Wang, P. Jian, and R. Jian, “Highly selective oxidation of styrene to benzaldehyde over a tailor-made cobalt oxide encapsulated zeolite catalyst,” J Colloid Interface Sci, vol. 517, pp. 144–154, May 2018, doi: 10.1016/j.jcis.2018.01.113.
L. B. Pierella, C. Saux, S. C. Caglieri, H. R. Bertorello, and P. G. Bercoff, “Catalytic activity and magnetic properties of Co-ZSM-5 zeolites prepared by different methods,” Appl Catal A Gen, vol. 347, no. 1, pp. 55–61, Sep. 2008, doi: 10.1016/j.apcata.2008.05.033.
F. Azzolina Jury, I. Polaert, L. B. Pierella, and L. Estel, “Optimized benzaldehyde production over a new Co-ZSM-11 catalyst: Reaction parameters effects and kinetics,” Catal Commun, vol. 46, pp. 6–10, Feb. 2014, doi: 10.1016/j.catcom.2013.11.020.
X. Guan, C. Duan, H. Wang, B. Lu, J. Zhao, and Q. Cai, “Tuneable oxidation of styrene to benzaldehyde and benzoic acid over Co/ZSM-5,” New Journal of Chemistry, vol. 45, no. 38, pp. 18192–18201, Oct. 2021, doi: 10.1039/d1nj03145g.
R. R. Nath, C. Nethravathi, and M. Rajamathi, “Hierarchically porous, biphasic, and C-doped TiO2for solar photocatalytic degradation of dyes and selective oxidation of benzyl alcohol,” ACS Omega, vol. 6, no. 18, pp. 12124–12132, 2021, doi: 10.1021/acsomega.1c00825.
Z. Topalian, B. I. Stefanov, C. G. Granqvist, and L. Österlund, “Adsorption and photo-oxidation of acetaldehyde on TiO2 and sulfate-modified TiO2: Studies by in situ FTIR spectroscopy and micro-kinetic modeling,” J Catal, vol. 307, pp. 265–274, 2013, doi: 10.1016/j.jcat.2013.08.004.
L. A. Al-Hajji, A. A. Ismail, M. Alsaidi, S. A. Ahmed, F. Almutawa, and A. Bumajdad, “Comparison of TiO2 nanowires and TiO2 nanoparticles for photodegradation of resorcinol as endocrine model,” Journal of Nanoparticle Research, vol. 22, no. 2, Jan. 2020, doi: 10.1007/s11051-020-4757-1.
X. Pan, Y. Zhao, S. Liu, C. L. Korzeniewski, S. Wang, and Z. Fan, “Comparing graphene-TiO 2 nanowire and graphene-TiO 2 nanoparticle composite photocatalysts,” ACS Appl Mater Interfaces, vol. 4, no. 8, pp. 3944–3950, Aug. 2012, doi: 10.1021/am300772t.
G. Gohari et al., “Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica,” Sci Rep, vol. 10, no. 1, Dec. 2020, doi: 10.1038/s41598-020-57794-1.
J. Jitputti, Y. Suzuki, and S. Yoshikawa, “Synthesis of TiO2 nanowires and their photocatalytic activity for hydrogen evolution,” Catal Commun, vol. 9, no. 6, pp. 1265–1271, Mar. 2008, doi: 10.1016/j.catcom.2007.11.016.
S. Al-Taweel, H. Saud, S. S. Al-Taweel, and H. R. Saud, “New route for synthesis of pure anatase TiO2 nanoparticles via utrasound-assisted sol-gel method Analytical chemistry View project New route for synthesis of pure anatase TiO 2 nanoparticles via utrasound-assisted sol-gel method,” Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, vol. 8, no. 2, pp. 620–626, 2016, [Online]. Available: www.jocpr.com
Y. Wang and J. Zhang, “Ultrafine TiO2(B) nanowires for ultrahigh-rate lithium-ion batteries,” Ionics (Kiel), vol. 26, no. 3, pp. 1159–1164, Mar. 2020, doi: 10.1007/s11581-019-03291-z.
L. S. Chougala, M. S. Yatnatti, R. K. Linganagoudar, R. R. Kamble, and J. S. Kadadevarmath, “A simple approach on synthesis of TiO2 nanoparticles and its application in dye sensitized solar cells,” Journal of Nano- and Electronic Physics, vol. 9, no. 4, 2017, doi: 10.21272/jnep.9(4).04005.
X. L. Zhang, Y. Chen, A. M. Cant, F. Huang, Y. B. Cheng, and R. Amal, “Crystalline TiO2 nanorod aggregates: Template-free fabrication and efficient light harvesting in dye-sensitized solar cell applications,” Particle and Particle Systems Characterization, vol. 30, no. 9, pp. 754–758, Sep. 2013, doi: 10.1002/ppsc.201300132.
W. Feng, G. Wu, L. Li, and N. Guan, “Solvent-free selective photocatalytic oxidation of benzyl alcohol over modified TiO2,” Green Chemistry, vol. 13, no. 11, pp. 3265–3272, Jan. 2011, doi: 10.1039/c1gc15595d.
C. M. Strollo and P. J. Ziemann, “Investigation of the formation of benzoyl peroxide, benzoic anhydride, and other potential aerosol products from gas-phase reactions of benzoylperoxy radicals,” Atmos Environ, vol. 130, pp. 202–210, Apr. 2016, doi: 10.1016/j.atmosenv.2016.01.011.
M. Sankar et al., “The benzaldehyde oxidation paradox explained by the interception of peroxy radical by benzyl alcohol,” Nat Commun, vol. 5, Feb. 2014, doi: 10.1038/ncomms4332.
License
Copyright (c) 2023 Sudirman Sudirman, Lalu Dimas Pratama Atmaja, Ari Jamhari Pratama, Emmy Yuanita, Ni Komang Tri Dharmayani, Maria Ulfa, Romel Hidayat

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish with ACA: Acta Chimica Asiana agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. This license allows authors to use all articles, data sets, graphics, and appendices in data mining applications, search engines, web sites, blogs, and other platforms by providing an appropriate reference. The journal allows the author(s) to hold the copyright without restrictions and will retain publishing rights without restrictions.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in ACA: Acta Chimica Asiana.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).





 Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia
Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia





