Effect of water-methanol binary solvent system in green synthesis of copper nanoparticles with tobacco leaf extract
Authors
Tomi Suharto , Sudirman , Maria Ulfa , Baiq Nila Sari Ningsih , Khoiria Nur Atika Putri , Panthong ThamsiriDOI:
10.29303/aca.v7i1.197Published:
2024-04-18Issue:
Vol. 7 No. 1 (2024)Keywords:
Copper Nanoparticles, Tobacco extract, Methanol-water systemArticles
Downloads
How to Cite
Downloads
Metrics
Abstract
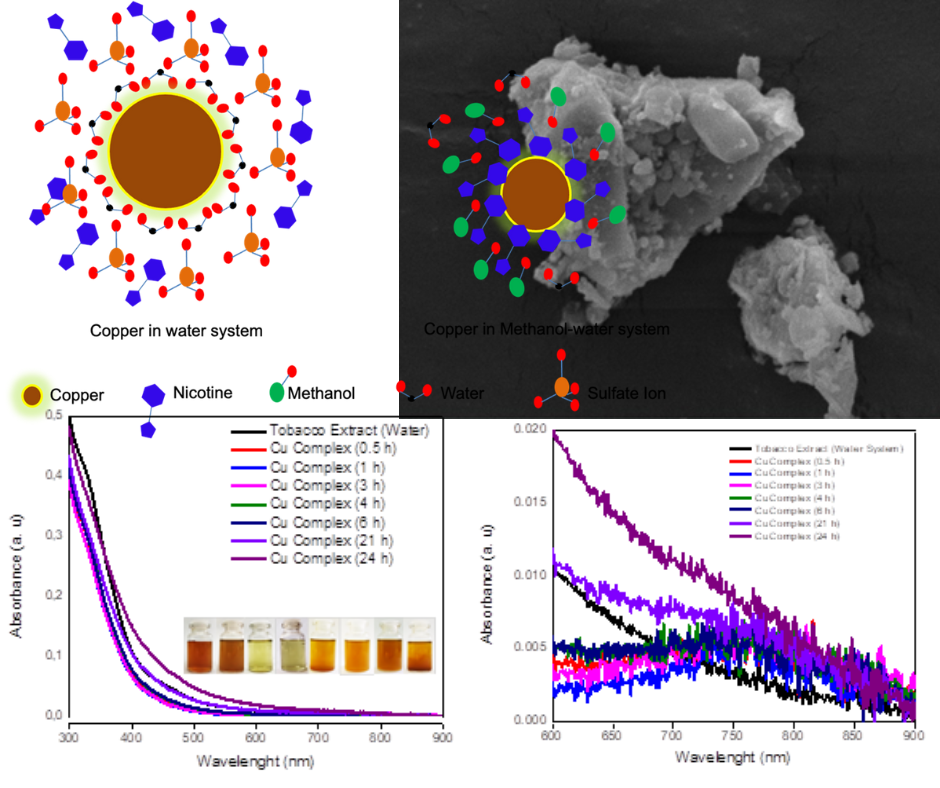
The increasing demand for environmentally friendly nanoparticle synthesis, as a viable strategy for pollution control, has prompted an exploration of green chemistry methods. This study meticulously investigates the synthesis of copper nanoparticles utilizing tobacco leaf extract obtained through both cold and hot extraction techniques. The primary focus lies in scrutinizing the impact of various solvents employed for plant extracts and metal ion solutions on reduction dynamics and particle size. The study employs UV-Vis and phytochemical analyses to discern differences in chemical composition and efficacy, particularly between the water and methanol-water systems, despite their resemblance in UV-Vis spectra. Intriguingly, the results unveil that the choice of solvent significantly influences the particle size distribution and stability of colloidal nanoparticles. The methanol-water system, in particular, yields smaller, more uniform particles compared to other solvent systems. This research sheds light on the pivotal role of solvent selection in nanoparticle synthesis, emphasizing its profound impact on both the reduction process and the resulting particle size distribution. The findings underscore the nuanced relationship between solvent choice and the characteristics of the synthesized nanoparticles, providing valuable insights for optimizing environmentally friendly synthesis methods. Ultimately, this study contributes to the growing body of knowledge on green chemistry approaches for nanoparticle synthesis with implications for pollution control and sustainable materials production.References
Chavali, M. S., & Nikolova, M. P. (2019). Metal oxide nanoparticles and their applications in nanotechnology. SN applied sciences, 1 (6), 607.
Mitchell, M. J., Billingsley, M. M., Haley, R. M., Wechsler, M. E., Peppas, N. A., & Langer, R. (2021). Engineering precision nanoparticles for
drug delivery. Nature reviews drug discovery, 20 (2), 101-124.
Singh, J., Dutta, T., Kim, K. H., Rawat, M., Samddar, P., & Kumar, P. (2018). ‘Green’synthesis of metals and their oxide nanoparticles: applications for environmental remediation. Journal of nanobiotechnology, 16, 1-24.
Andualem, W. W., Sabir, F. K., Mohammed, E. T., Belay, H. H., & Gonfa, B. A. (2020). Synthesis of copper oxide nanoparticles using plant leaf extract of Catha edulis and its antibacterial activity. Journal of Nanotechnology, 2020, 1-10.
Alhalili, Z. (2022). Green synthesis of copper oxide nanoparticles CuO NPs from Eucalyptus Globoulus leaf extract: Adsorption and design of experiments. Arabian Journal of Chemistry, 15 (5), 103739..
Mali, S. C., Dhaka, A., Githala, C. K., & Trivedi, R. (2020). Green synthesis of copper nanoparticles using Celastrus paniculatus Willd. leaf extract and their photocatalytic and antifungal properties. Biotechnology Reports, 27, e00518.
Abosede, O. O., & Obiyenwa, G. K. (2022). Green synthesis of copper oxide nanoparticles using Justicia Carnea. Int. J. Chem. Stud, 10 (2), 21-25.
Manzoor, M. A., Shah, I. H., Sabir, I. A., Ahmad, A., Albasher, G., Dar, A. A., & Shakoor, A. (2023). Environmental sustainable: biogenic copper oxide nanoparticles as nano-pesticides for investigating bioactivities against phytopathogens. Environmental Research, 231, 115941.
Murugappan, G., & Sreeram, K. J. (2021). Nano-biocatalyst: Bi-functionalization of protease and amylase on copper oxide nanoparticles. Colloids and Surfaces B: Biointerfaces, 197, 111386.
Priya, M., Venkatesan, R., Deepa, S., Sana, S. S., Arumugam, S., Karami, A. M., & Kim, S. C. (2023). Green synthesis, characterization, antibacterial, and antifungal activity of copper oxide nanoparticles derived from Morinda citrifolia leaf extract. Scientific Reports, 13 (1), 18838.
Nzilu, D. M., Madivoli, E. S., Makhanu, D. S., Wanakai, S. I., Kiprono, G. K., & Kareru, P. G. (2023). Green synthesis of copper oxide nanoparticles and its efficiency in degradation of rifampicin antibiotic. Scientific Reports, 13 (1), 14030.
Bezuneh, T. T. (2015). Phytochemistry and antimicrobial activity of Parthenium hysterophorus L.: A review. Sci. J. Anal. Chem, 3 (3), 30.
Djajadi, D. (2015). Tobacco diversity in Indonesia. Berkala Penelitian Hayati, 20 (2), 27-32.
Clayton, P. M., Vas, C. A., Bui, T. T., Drake, A. F., & Mc Adam, K. (2013). Spectroscopic studies on nicotine and nornicotine in the UV region. Chirality, 25 (5), 288-293.
Aravinda, C. L., Mayanna, S. M., & Muralidharan, V. S. (2000). Electrochemical behaviour of alkaline copper complexes. Journal of Chemical Sciences, 112, 543-550.
Rahardjo, S. B., Saraswati, T. E., Masykur, A., Finantrena, N. N. F., & Syaima, H. (2018). Synthesis and characterization of tetrakis (2-amino-3-methylpyridine) copper (II) sulfate tetrahydrate. In IOP Conference Series: Materials Science and Engineering (Vol. 349, No. 1, p. 012056). IOP Publishing.
Fazary, A. E., Ju, Y. H., Fawy, K. F., Al-Shihri, A. S., Bani-Fwaz, M. Z., Alfaifi, M. Y., ... & Abd-Rabboh, H. S. (2017). Nicotine–Metal ion interactions in solutions: Potentiometric, cyclic voltammetry investigations and quantum chemical calculations. The Journal of Chemical Thermodynamics, 112, 283-292.
Kaowphong, S., Nakashima, K., Petrykin, V., Thongtem, S., & Kakihana, M. (2009). Methanol–Water System for Solvothermal Synthesis of YVO4: Eu with High Photoluminescent Intensity. Journal of the American Ceramic Society, 92, S16-S20.
Masakke, Y., & Sulfikar, R. M. (2015). Biosynthesis of silver nanoparticles using methanol extract of mangosteen leaves (Garcinia mangostana L.). Sainsmat: Scientific Journal of Natural Sciences, 4 (1), 28-41.
Taba, P., Parmitha, N. Y., & Kasim, S. (2019). Synthesis of silver nanoparticles using bay leaf extract (Syzygium polyanthum) as bioreductor and test of its activity as antioxidant. Indonesian Journal of Chemical Research, 7 (1), 51-60.
License
Copyright (c) 2024 Tomi Suharto, Sudirman, Maria Ulfa, Baiq Nila Sari Ningsih, Khoiria Nur Atika Putri, Panthong Thamsiri

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish with ACA: Acta Chimica Asiana agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. This license allows authors to use all articles, data sets, graphics, and appendices in data mining applications, search engines, web sites, blogs, and other platforms by providing an appropriate reference. The journal allows the author(s) to hold the copyright without restrictions and will retain publishing rights without restrictions.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in ACA: Acta Chimica Asiana.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).





 Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia
Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia





