Exploration of secondary metabolite profile in the n-hexane fraction of Rhizophora mucronata, Avicennia marina, and Sonneratia alba
Authors
Raehanul Maziya , Lina Permatasari , Rizqa Fersiyana Deccati , Handa Muliasari , Fania Rahman , Zulfiana Fatianingrum Annas , Rahula Vijja SammantaDOI:
10.29303/aca.v8i1.231Published:
2025-05-31Issue:
Vol. 8 No. 1 (2025)Keywords:
Rhizophora mucronata, Sonneratia alba, Avicennia marina, mangrove leaves, Gas Chromatography-MassArticles
Downloads
How to Cite
Downloads
Metrics
Abstract
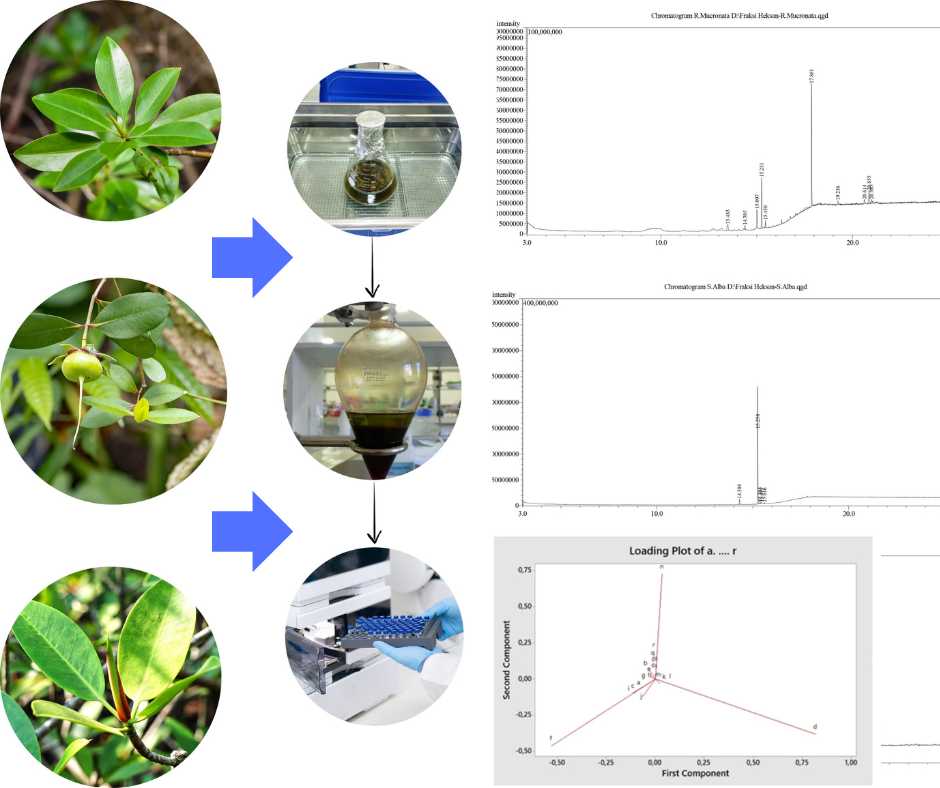
Indonesia is a maritime and archipelagic country with an ocean area of almost two-thirds of its total area, with a coastline stretching 99.123 km from Sabang to Merauke. According to Indonesian Law Number 1 of 2014, it is mentioned that one of the most important biological resources of the coast is mangroves. Some mangrove species commonly found on Lombok Island are Rhizophora mucronata, Sonneratia alba, and Avicennia marina. However, there has not been much exploration of the compound content in these mangroves. Therefore, this study aimed to identify the secondary metabolites of the n-hexane fraction of the three mangrove species using Gas Chromatography-Mass Spectrometry (GC-MS). The leaves of each mangrove species were extracted by sonication method using 96% ethanol solvent, followed by multistage fractionation using n-hexane and water. GC-MS analyzed the n-hexane fraction of each mangrove species. The GC-MS analysis revealed that in the n-hexane fraction of mangrove leaves Rhizophora mucronata and Avicennia marina there were 10 compounds, while Sonneratia alba obtained five compounds. The compounds with the highest intensity in the n-hexane fraction of mangrove leaves of Rhizophora mucronata, Sonneratia alba, and Avicennia marina were squalene (41.71%), ethyl oleate (87.53%), and ethyl oleate (44.02%), respectively. Squalene was reported to have antioxidant and anticancer activities. The ethyl oleate was reported to have bactericidal activity on gram-positive and negative bacteria. The three types of mangrove leaves can be an alternative source of medicine
References
Maulana, M., Moehammad, A., & Janu, F. A. (2017). Analisis Pengaruh Perubahan Garis Pantai Terhadap Batas Pengelolaan Wilayah Laut Provinsi Jawa Timur Dan Provinsi Bali Di Selat Bali. Jurnal Geodesi Undip, 55(4), 233–242.
Pemerintah Republik Indonesia. (2014). Undang-Undang RI Nomor 1 Tahun 2014 Tentang Perubahan Atas Undang-Undang Nomor 27 Tahun 2007 Tentang Pengelolaan Wilayah Pesisir Dan Pulau-Pulau Kecil. Lembaran Negara Republik Indonesia, 8.
Husuna, R., Wantasen, A. S., & Rondonuwu, A. B. (2019). Struktur Komunitas Mangrove di Pantai Tabulo Selatan Kabupaten Boalemo. Jurnal Ilmiah Platax, 7(1), 309–319.
Rahmani, A. V., Idrus, A. Al, & Mertha, I. G. (2023). The Structure of Mangrove Community in Regional Marine Conservation Area Gili Sulat West Nusa Tenggara. Jurnal Biologi Tropis, 23(1), 42–51. https://doi.org/10.29303/jbt.v23i1.4597
Rahman, F. A. (2022). Ekologi mangrove Pulau Lombok (Mu. L. Irrubai (ed.); 1st ed.). UIN Mataram Press.
Pambudi, D. B., & Haryoto. (2022). Efektivitas Farmakologi Senyawa Aktif Tumbuhan Mangrove Yang Hidup Di Indonesia. Jurnal Ilmiah Kesehatan, 15(1), 39–57. https://doi.org/10.48144/jiks.v15i1.625
Setyawan, A. D., Ragavan, P., Basyuni, M., & Sarno, S. (2022). Review: Rhizophora mucronata as source of foods and medicines. International Journal of Bonorowo Wetlands, 9(1), 42–55. https://doi.org/10.13057/bonorowo/w090105
Abubakar, S., Kadir, asykhur A., Wibowo, E. S., & Nebuchadnezzar Akbar. (2019). Manfaat Mangrove Bagi Peruntukan Sediaan Farmasitika Di Desa Mamuya Kecamatan Galela Timur Kabupaten Halmahera Timur (Tinjauan Etnofarmakologis). Jurnal Enggano, 4(1), 12–25.
Putri, R. R., Hasanah, R., & Kusimaningrum, I. (2016). Uji Aktivitas Antibakteri dan Uji Fitokimia Ekstrak Daun Mangrove Sonneratia alba Anti-bacterial. Aquawarman Jurnal Sains Dan Teknologi Akuakultur, 2(1), 43–50.
Fitri, A., & Usman. (2021). Aktivitas Antioksidan Ekstrak Metanol Daun Mangrove (Avicennia Marina). Prosiding Seminar Nasional Kimia, 1, 12–17.
Muliyana. (2020). Kajian Pemanfaatan Ekstrak Daun Bakau Sebagai Agen Antifertilitas. In Jurusan Pendidikan IPA-Biologi Fakultas Tarbiyah dan Keguruan Universitas Islam Negeri Mataram. https://doi.org/10.1016/j.jnc.2020.125798%0Ahttps://doi.org/10.1016/j.smr.2020.02.002%0Ahttp://www.ncbi.nlm.nih.gov/pubmed/810049%0Ahttp://doi.wiley.com/10.1002/anie.197505391%0Ahttp://www.sciencedirect.com/science/article/pii/B9780857090409500205%0Ahttp:
Adhikari, A., Ray, M., Das, A. K., & Sur, T. K. (2016). Antidiabetic and antioxidant activity of Rhizophora mucronata leaves (Indian sundarban mangrove): An in vitro and in vivo study. AYU (An International Quarterly Journal of Research in Ayurveda), 37(1), 76. https://doi.org/10.4103/ayu.ayu_182_15
Karundeng, E. D. B., Hanizar, E., & Sari, D. N. R. (2022). Potensi Ekstrak Daun Rhizophora mucronata Sebagai Antibakteri Pada Staphylococcus aureus. BIOSAPPHIRE: Jurnal Biologi Dan Diversitas, 1(1), 10–18. https://doi.org/10.31537/biosapphire.v1i1.642
Kaur, S., Syed Ali, M., Anuradha, V., Suganya, V., Ashashalini, A., & Bhuvana, P. (2018). In vitro anti-inflammatory activity of mangrove plant Rhizophora mucronata Lam. (Malpighiales: Rhizophoraceae). Brazilian Journal of Biological Sciences, 5(10), 417–426. https://doi.org/10.21472/bjbs.051018
Mulyani, Y., Syaputra, N. D., Dewi, K. C., Lili, W., & Agung, M. U. K. (2019). Total phenolic , flavonoid content and antioxidant capacity of stem bark , root , and leaves methanolic extract of Rhizophora mucronata Lam . World News of Natural Science, 26(August), 118–127.
Pertiwi, R., Yudha, S. S., Wibowo, R. H., Notriawan, D., Nasution, R. P., & Azhar, A. W. (2024). Aktivitas Antibakteri Ekstrak Daun Mangrove (Rhizophora mucronata) pada Bakteri Helicobacter pylori Penyebab Tukak Lambung. Bioscientist: Jurnal Ilmiah Biologi, 12(1), 202–209.
Ridlo, A., Pramesti, R., Koesoemadji, K., Supriyantini, E., & Soenardjo, N. (2017). Aktivitas Antioksidan Ekstrak Daun Mangrove Rhizopora mucronata. Buletin Oseanografi Marina, 6(2), 110. https://doi.org/10.14710/buloma.v6i2.16555
Suganthy, N., & Devi, K. P. (2016). In vitro antioxidant and anti-cholinesterase activities of Rhizophora mucronata. Pharmaceutical Biology, 54(1), 118–129. https://doi.org/10.3109/13880209.2015.1017886
Usman, U., Masruhim, M. A., Kusumaningtiyas, P., Erwin, E., & Bulan, D. E. (2023). Antioxidant and Antidiabetic from Rhizophora mucronata Derived from Sambera. Tropical Journal of Natural Product Research, 7(10), 4921–4926.
Binuni, R., Maarisit, W., Hariyadi, H., & Saroinsong, Y. (2020). Uji Aktivitas Antioksidan Ekstrak Daun Mangrove Sonneratia alba Dari Kecamatan Tagulandang, Sulawesi Utara Menggunakan Metode DPPH. Biofarmasetikal Tropis, 3(1), 79–85. https://doi.org/10.55724/j.biofar.trop.v3i1.260
Musa, W. J. ., Bialangi, N., Situmeang, B., & Silaban, S. (2019). Triterpenoid compound from metanol extract of mangrove leaves (Sonneratia alba) and anti-cholesterol activity test. Jurnal Pendidikan Kimia, 11(1), 18–23. https://doi.org/10.24114/jpkim.v11i1.13124
Asad, S., Nesa, L., Deepa, K. N., Sohel, D., & Kawsar. (2017). Analgesic, Anti-Inflammatory and CNS Depressant Activities of Methanolic Extract of Sonneratia alba Leaves in Mice. Natural Products Chemistry & Research, 05(05), 1–7. https://doi.org/10.4172/2329-6836.1000277
Thu, N. T. H., Khanh, L. P., Duy, N. T., Chanh, N. K., Phung, N. K. P., & Hansen, P. E. (2011). Chemical Constituents From Leaves of Sonneratia Alba J.E. Smith (Sonneratiaceae). Science and Technology Development Journal, 14(4), 11–17. https://doi.org/10.32508/stdj.v14i4.2043
Annas, Z. F., Deccati, R. F., Permatasari, L., & Mukhlishah, N. R. I. (2023). Penentuan Kadar Flavonoid Total Ekstrak dan Fraksi-Fraksi Daun Mangrove (Avicennia marina). Program Studi Farmasi Fakultas Kedokteran Dan Ilmu Kesehatan Universitas Mataram.
Roy, A., Das, S., Chatterjee, I., Roy, S., & Chakraborty, R. (2022). Anti-inflammatory Effects of Different Dietary Antioxidants BT - Plant Antioxidants and Health (H. M. Ekiert, K. G. Ramawat, & J. Arora (eds.); pp. 573–597). Springer International Publishing. https://doi.org/10.1007/978-3-030-78160-6_20
Sammanta, R. V., Muliasari, H., & Mukhlishah, N. R. I. (2023). Penentuan Kadar Flavonoid Total Ekstrak dan Fraksi-Fraksi Daun Mangrove ( Rhizopora mucronata ). Proceedings of the Institution of Mechanical Engineers, Part J: Journal of Engineering Tribology, 1(1), 1–25.
Annas, Z. F., Deccati, R. F., Permatasari, L., & Mukhlishah, N. R. I. (2023). Penentuan Kadar Flavonoid Total Ekstrak dan Fraksi-Fraksi Daun Mangrove (Avicennia marina). Program Studi Farmasi Fakultas Kedokteran Dan Ilmu Kesehatan Universitas Mataram.
Rahman, F. (2023). Penentuan Kadar Flavonoid Total Ekstrak dan Fraksi-fraksi Daun Mangrove (Sonneratia alba). Universitas Mataram.
Sianturi, G. L. R., Trisnawati, E. W., Koketsu, M., & Suryanti, V. (2023). Chemical constituents and antioxidant activity of Britton’s wild petunia (Ruellia brittoniana) flower. Biodiversitas, 24(7), 3665–3672. https://doi.org/10.13057/biodiv/d240703
Amudha, P., Jayalakshmi, M., Pushpabharathi, N., & Vanitha, V. (2018). Identification of bioactive components in enhalus acoroides seagrass extract by gas chromatography–mass spectrometry. Asian Journal of Pharmaceutical and Clinical Research, 11(10), 313–317. https://doi.org/10.22159/ajpcr.2018.v11i10.25577
Heng, Y. W., Ban, J. J., Khoo, K. S., & Sit, N. W. (2020). Biological activities and phytochemical content of the rhizome hairs of Cibotium barometz (Cibotiaceae). Industrial Crops and Products, 153(April), 112612. https://doi.org/10.1016/j.indcrop.2020.112612
Alencar, M. V. O. B., Islam, M. T., Ali, E. S., Santos, J. V. O., Paz, M. F. C. J., Sousa, J. M. C., Dantas, S. M. M. M., Mishra, S. K., & Cavalcante, A. A. C. M. (2018). Association of Phytol with Toxic and Cytotoxic Activities in an Antitumoral Perspective: A Meta-Analysis and Systemic Review. Anti-Cancer Agents in Medicinal Chemistry, 18(13), 1828–1837. https://doi.org/10.2174/1871520618666180821113830
Al-Mohammadi, A.-R., Ibrahim, R. A., Mustofa, A. H., Ismaiel, A. A., Zeid, A. A., & Gamal, E. (2021). Chemical Constitution and Antimicrobial Activity of Kefir Fermented Beverage. Molecules, 26(9), 1–19.
Zhao, J., Shan, T., Huang, Y., Liu, X., Gao, X., Wang, M., Jiang, W., & Zhou, L. (2010). Chemical Composition and In Vitro Antimicrobial Activity of the Volatile Oils from Gliomastix murorum and Pichia guilliermondii, Two Endophytic Fungi in Paris polyphylla var. yunnanensis Jianglinural Product Communications. NPC Natural Product Communications, 4(11), 1491–1496.
Katz, D. H., Marcelletti, J. F., Khalil, M. H., Pope, L. E., & Katz, L. R. (1991). Antiviral activity of 1-docosanol, an inhibitor of lipid-enveloped viruses including herpes simplexfile. Proceedings of the National Academy of Sciences of the United States of America, 88(23), 10825–10829. https://doi.org/10.1073/pnas.88.23.10825
Dhankhar, S., Dhankhar, S., Ruhil, S., Balhara, M., Malik, V., & Chhillar, A. K. (2014). Isolation and biological evaluation of novel Tetracosahexaene hexamethyl, an acyclic triterpenoids derivatives and antioxidant from Justicia adhatoda. Combinatorial Chemistry & High Throughput Screening, 17(8), 723–732. https://doi.org/10.2174/1386207317666140708091552
Gautam, V., Kohli, S. K., Arora, S., Bhardwaj, R., Kazi, M., Ahmad, A., Raish, M., Ganaie, M. A., & Ahmad, P. (2018). Antioxidant and antimutagenic activities of different fractions from the leaves of rhododendron arboreum sm. And their gc-ms profiling. Molecules, 23(9), 1–12. https://doi.org/10.3390/molecules23092239
Rizvi, S., Raza, S. T., Ahmed, F., Ahmad, A., Abbas, S., & Mahdi, F. (2014). The role of Vitamin E in human health and some diseases. Sultan Qaboos University Medical Journal, 14(2), 157–165.
Chen, D. F., Zhang, H. L., Du, S. H., Li, H., Zhou, J. H., Li, Y. W., Zeng, H. P., & Hua, Z. C. (2010). Cholesterol myristate suppresses the apoptosis of mesenchymal stem cells via upregulation of inhibitor of differentiation. Steroids, 75(13–14), 1119–1126. https://doi.org/10.1016/J.STEROIDS.2010.07.009
National Center for Biotechnology Information. (2024). Squalene. PubChem Compound Summary for CID 638072. https://pubchem.ncbi.nlm.nih.gov/compound/Squalene
Zhang, P., Liu, N., Xue, M., Zhang, M., Xiao, Z., Xu, C., Fan, Y., Liu, W., Qiu, J., Zhang, Q., & Zhou, Y. (2023). Anti-Inflammatory and Antioxidant Properties of Squalene in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio). International Journal of Molecular Sciences, 24(10). https://doi.org/10.3390/ijms24108518
Micera, M., Botto, A., Geddo, F., Antoniotti, S., Bertea, C. M., Levi, R., Gallo, M. P., & Querio, G. (2020). Squalene: More than a step toward sterols. Antioxidants, 9(8), 1–14. https://doi.org/10.3390/antiox9080688
Bhat, M. P., Rudrappa, M., Hugar, A., Gunagambhire, P. V., Suresh Kumar, R., Nayaka, S., Almansour, A. I., & Perumal, K. (2023). In-vitro investigation on the biological activities of squalene derived from the soil fungus Talaromyces pinophilus. Heliyon, 9(11), e21461. https://doi.org/10.1016/j.heliyon.2023.e21461
Cárdeno, A., Aparicio-Soto, M., Montserrat-de la Paz, S., Bermudez, B., Muriana, F. J. G., & Alarcón-de-la-Lastra, C. (2015). Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. Journal of Functional Foods, 14, 779–790. https://doi.org/10.1016/j.jff.2015.03.009
Cheng, Z., Abayasekara, D. R. E., Elmes, M., Kirkup, S., & Wathes, D. C. (2015). Effect of oleic acid supplementation on prostaglandin production in maternal endometrial and fetal allantochorion cells isolated from late gestation ewes. Placenta, 36(9), 1011–1017. https://doi.org/10.1016/j.placenta.2015.07.128
Jesica, F., & Friadi, A. (2019). Hubungan Kadar Kortisol Dan Prostaglandin Maternal Dengan Persalinan Preterm Dan Aterm. Jurnal Ilmu Keperawatan Dan Kebidanan, 10(1),
Krishnaveni, M., Banu, C. R., Kalaivani, M., & Krishnakumari, G. (2015). GC-MS Analysis of Phytochemicals in Muntingia Calabura L, Antimicrobiasl Assay. SJIF Journal, 4(4), 1855–1859. https://doi.org/10.20959/wjpr202317-29690
Akin-Osanaiye, C. B., Gabriel, A. F., & Alebiosu, R. A. (2011). Characterization and Antimicrobial Screening of Ethyl Oleat Isolated from Phyllanthus Amarus (Sehum and Thonn). Annals of Biological Research, 2(2), 298–305.
Zheng, C. J., Yoo, J. S., Lee, T. G., Cho, H. Y., Kim, Y. H., & Kim, W. G. (2005). Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Letters, 579(23), 5157–5162. https://doi.org/10.1016/J.FEBSLET.2005.08.028
An, K., Fu, M., Zhang, H., Tang, D., Xu, Y., & Xiao, G. (2019). Effect of ethyl oleate pretreatment on blueberry (Vaccinium corymbosum L.): drying kinetics, antioxidant activity, and structure of wax layer. Journal of Food Science and Technology, 56(2), 783–791. https://doi.org/10.1007/s13197-018-3538-7
Sagna, A., Nair, R. V. R., Hulyalkar, N., Rajasekharan, S., Nair, V. T. G., Sivakumar, K. C., Suja, S. R., Baby, S., & Sreekumar, E. (2023). Ethyl palmitate, an anti-chikungunya virus principle from Sauropus androgynus, a medicinal plant used to alleviate fever in ethnomedicine. Journal of Ethnopharmacology, 309, 116366. https://doi.org/10.1016/j.jep.2023.116366
Saeed, N. M., El-Demerdash, E., Abdel-Rahman, H. M., Algandaby, M. M., Al-Abbasi, F. A., & Abdel-Naim, A. B. (2012). Anti-inflammatory activity of methyl palmitate and ethyl palmitate in different experimental rat models. Toxicology and Applied Pharmacology, 264(1), 84–93. https://doi.org/10.1016/j.taap.2012.07.020
Muflihunna, A., Mu’Nisa, A., Hala, Y., & Hasri. (2021). Gas Chromatography-Mass Spectrometry (GC-MS) Analysis and Antioxidant Activity of Sea-Cucumber (Holothurian atra and Holothurian edulis) from Selayar Island. Journal of Physics: Conference Series, 1752(1). https://doi.org/10.1088/1742-6596/1752/1/012057
Park, S. Y., Seetharaman, R., Ko, M. J., Kim, D. Y., Kim, T. H., Yoon, M. K., Kwak, J. H., Lee, S. J., Bae, Y. S., & Choi, Y. W. (2014). Ethyl linoleate from garlic attenuates lipopolysaccharide-induced pro-inflammatory cytokine production by inducing heme oxygenase-1 in RAW264.7 cells. International Immunopharmacology, 19(2), 253–261. https://doi.org/10.1016/j.intimp.2014.01.017
Arriza, N. (2022). Pengaruh Teh Lidah Buaya (Aloe Vera) Terhadap Penanganan Konstipasi Dan Keamanan Pertumbuhan Fetus Pada Mencit Bunting. In Sekolah Pascasarjana Program Studi Magister Kebidanan Universitas Hasanuddin Makasar.
Jenecius, A., Uthayakumari, F., & Mohan, V. (2012). Gc-Ms Determination of Bioactive Components of Sauropus Bacciformis Blume (Euphorbiaceae). Journal of Current Chemical and Pharmaceutical Science, 2(January 2012), 347–358.
Khan, Z., Nath, N., Rauf, A., Emran, T. Bin, Mitra, S., Islam, F., Chandran, D., Barua, J., Khandaker, M. U., Idris, A. M., Wilairatana, P., & Thiruvengadam, M. (2022). Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chemico-Biological Interactions, 365, 110117. https://doi.org/10.1016/J.CBI.2022.110117
De Almeida, P. D. O., Boleti, A. P. D. A., Rüdiger, A. L., Lourenço, G. A., Da Veiga Junior, V. F., & Lima, E. S. (2015). Anti-inflammatory activity of triterpenes isolated from Protium paniculatum oil-resins. Evidence-Based Complementary and Alternative Medicine, 2015, 1–10. https://doi.org/10.1155/2015/293768
Ferreira, R. G. S., Silva, W. F., Veiga, V. F., Lima, Á. A. N., & Lima, E. S. (2017). Physicochemical characterization and biological activities of the triterpenic mixture α,β-amyrenone. Molecules, 22(2), 1–9. https://doi.org/10.3390/molecules22020298
Raman, V. B., Samuel, L. A., Saradhi, P. M., Rao, N. B., Krishna, N. V. A., Sudhakar, M., & Radhakrishnan, T. M. (2012). Academic Sciences Asian Journal of Pharmaceutical and Clinical Research. Asian Journal of Pharmaceutical and Clinical Research, 5(2), 99–106.
Gairola, K., Gururani, S., Kumar, R., Prakash, O., Agrawal, S., & Dubey, S. K. (2022). Composition, Antioxidant and Anti-inflammatory activities of Hexane and Methanol extracts of Acmella uliginosa from Terai region of Uttarakhand. Brazilian Journal of Pharmaceutical Sciences, 58. https://doi.org/10.1590/s2175-97902022e20353
Saddiq, A. A., Tag, H. M., Doleib, N. M., Salman, A. S., & Hagagy, N. (2022). Antimicrobial, Antigenotoxicity, and Characterization of Calotropis procera and Its Rhizosphere-Inhabiting Actinobacteria: In Vitro and In Vivo Studies. Molecules, 27(10), 1–19. https://doi.org/10.3390/molecules27103123
Paputungan, Z., Wonggo, D., & Kaseger, B. E. (2017). Uji Fitokimia dan Aktivitas Antioksidan Buah Mangrove Sonneratia alba di Desa Nunuk Kecamatan Pinolosian Kabupaten Bolaang Mongondow Selatan. Media Teknologi Hasil Perikanan, 5(3), 96. https://doi.org/10.35800/mthp.5.3.2017.16866
Sharma, P., Jha, A. B., Dubey, R. S., & Pessarakli, M. (2012). Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. Journal of Botany, 2012, 1–26. https://doi.org/10.1155/2012/217037
Nizam, A., Meera, S. P., & Kumar, A. (2022). Genetic and molecular mechanisms underlying mangrove adaptations to intertidal environments. IScience, 25(1), 103547. https://doi.org/https://doi.org/10.1016/j.isci.2021.103547
Jithesh, M. N., Prashanth, S. R., Sivaprakash, K. R., & Parida, A. (2006). Monitoring expression profiles of antioxidant genes to salinity, iron, oxidative, light and hyperosmotic stresses in the highly salt tolerant grey mangrove, Avicennia marina (Forsk.) Vierh. by mRNA analysis. Plant Cell Reports, 25(8), 865–876. https://doi.org/10.1007/s00299-006-0127-4
Wallace, W. E., & Moorthy, A. S. (2023). NIST Mass Spectrometry Data Center standard reference libraries and software tools: Application to seized drug analysis. Journal of Forensic Sciences, 68(5), 1484–1493. https://doi.org/10.1111/1556-4029.15284
Oh, S.-W., Imran, M., Kim, E.-H., Park, S.-Y., Lee, S.-G., Park, H.-M., Jung, J.-W., & Ryu, T.-H. (2023). Approach strategies and application of metabolomics to biotechnology in plants. Frontiers in Plant Science, 14, 1192235. https://doi.org/10.3389/fpls.2023.1192235
Deborde, C., Moing, A., Roch, L., Jacob, D., Rolin, D., & Giraudeau, P. (2017). Plant metabolism as studied by NMR spectroscopy. Progress in Nuclear Magnetic Resonance Spectroscopy, 102–103, 61–97. https://doi.org/10.1016/j.pnmrs.2017.05.001
Ren, J.-L., Zhang, A.-H., Kong, L., & Wang, X.-J. (2018). Advances in mass spectrometry-based metabolomics for investigation of metabolites. RSC Advances, 8(40), 22335–22350. https://doi.org/10.1039/c8ra01574k
Saputri, L. O., Nurhidayati, Nurhidayati, Harahap, H. S., Zubaidi, F. F., Rivarti, A. W., & Permatasari, L. (2024). Principal compounds and antioxidant activity of various sample particle sizes of sea urchin shells from coastal area of Lombok Island. Research Journal of Pharmacy and Technology, 17(12).
Caesar, L. K., Kvalheim, O. M., & Cech, N. B. (2018). Hierarchical cluster analysis of technical replicates to identify interferents in untargeted mass spectrometry metabolomics. Analytica Chimica Acta, 1021, 69–77. https://doi.org/10.1016/j.aca.2018.03.013
License
Copyright (c) 2025 Raehanul Maziya, Lina Permatasari, Rizqa Fersiyana Deccati, Handa Muliasari, Fania Rahman, Zulfiana Fatianingrum Annas, Rahula Vijja Sammanta

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish with ACA: Acta Chimica Asiana agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. This license allows authors to use all articles, data sets, graphics, and appendices in data mining applications, search engines, web sites, blogs, and other platforms by providing an appropriate reference. The journal allows the author(s) to hold the copyright without restrictions and will retain publishing rights without restrictions.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in ACA: Acta Chimica Asiana.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).





 Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia
Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia





