Hydrothermal synthesis of crystalline Aluminium(III)-Tartrate: effect of tartrate type and molar ratio
Authors
Yuniar Ponco Prananto , Danar Purwonugroho , M. Naufal Tsaqif Dzakwan , Tutik Setianingsih , Nidatul SyarifahDOI:
10.29303/aca.v8i1.244Published:
2025-05-31Issue:
Vol. 8 No. 1 (2025)Keywords:
metal complex, mordent, crystalline material, precursor, mol ratioArticles
Downloads
How to Cite
Downloads
Metrics
Abstract
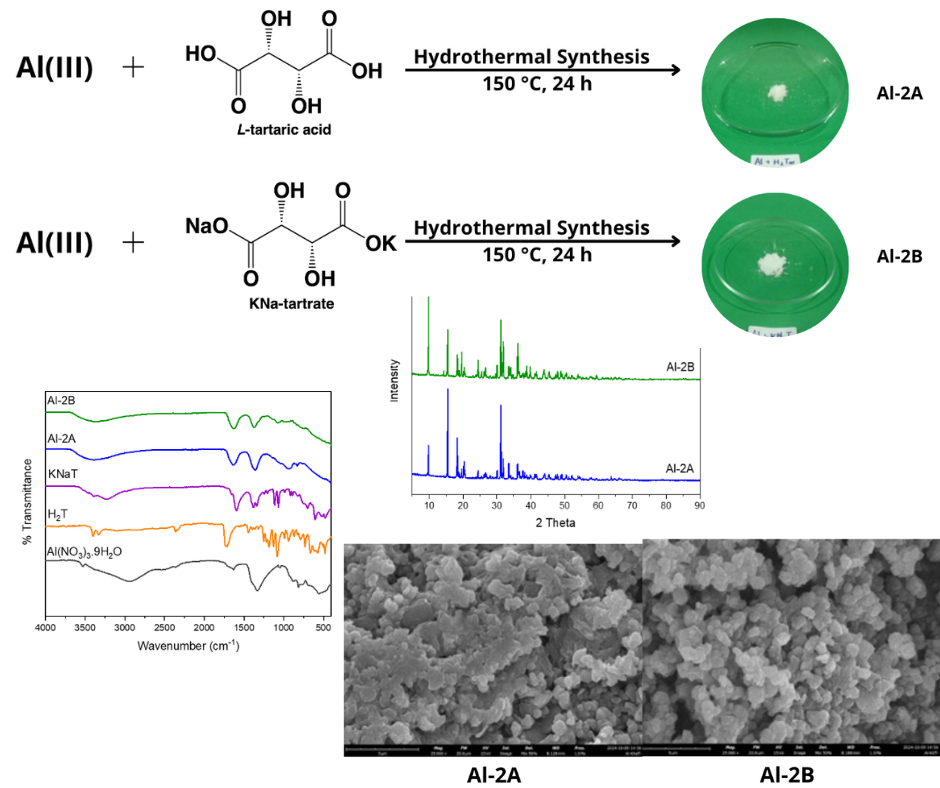
Aluminium(III)-tartrate (Al-T) complex is a compound that commonly used as mordent in textile dyeing. This complex is soluble in hot water; thus, information on the isolation of crystallised Al-T is limited. Isolation of crystallised Al-T is needed to gain a high purity complex for further application in the textile industry. This study aims to synthesize and isolate crystalline complex of Al-T. Hydrothermal method was used to obtain the targeted complex. Effects of tartrate precursor and Al(III):tartrate mol ratio in the synthesis of Al-T complex were also investigated. The synthesis was done at 150 °C for 24 hours in several Al(III):tartrate mol ratios (1:2, 2:1, and 2:3) using two different tartrate precursors, namely L-tartaric acid and KNa-tartrate. The synthesized complexes were identified by infrared spectroscopy and powder-XRD analyses, and then further characterised by UV Vis - DRS, DTA-TGA, and SEM. Experimental data shows that the mol ratio affects the precipitation of the Al-T complex, in which a white crystalline solid was only precipitated out from the 2:1 reaction by both tartrate precursors. Different tartrate precursors used in the synthesis may alter the crystallization and result in an Al-T complex with slightly different thermal decomposition profile, UV-Vis DRS spectra profile, and different yield due to the different nature of the tartrate precursor. This finding is expected to support the possibility of Al-T mass production as mordent in textile dyeing
References
Jafar, N., Nurhawaisyah, S. R., Widodo, S., Chalik, C. A., & Wakila, M. H. (2023). Mineralogical Study of Bauxite of Kenco Area, Landak District, West Kalimantan Province, Indonesia. IOP Conference Series: Earth and Environmental Science, 1134(1), 012025. https://doi.org/10.1088/1755-1315/1134/1/012025
Kementerian ESDM Indonesia. (2020). Booklet Tambang Bauksit 2020. https://www.esdm.go.id/id/booklet/booklet-tambang-bauksit-2020. Retrieved December 27, 2024.
Syarwani, A. & Prananto, Y. P. (2023). Literature Study on the Screening of Al-MOFs Porous Material for Dimethyl Ether (DME) Gas Purification. Jurnal Integrasi Proses, 12(2), 55-65. https://dx.doi.org/10.36055/jip.v12i2.17485
Joshi, J. N., Moran, C. M., Feininger, H. P., Dow, J. M., & Walton, K. S. (2019). Household Aluminum Products as Insoluble Precursors for Directed Growth of Metal–Organic Frameworks. Crystal Growth & Design, 19(9), 5097–5104.
Ding, M., Cai, X., & Jiang, H. L. (2019). Improving MOF Stability: Approaches and Applications. Chemical Science, 10(44), 10209-10230. DOI: 10.1039/C9SC03916C
Samanidou, V. F., & Deliyannni, E. A. (2020). Metal Organic Frameworks: Synthesis and Application. Molecules, 25, 960, 1. https://doi.org/10.3390/molecules25040960
Yusuf, V. F., Malek, N. I., & Kailasa, S. K. (2022). Review on Metal–Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment. ACS Omega, 7(49), 44507–44531. https://doi.org/10.1021/acsomega.2c05310
Majumder, M., Choudhary, R. B., Thakur, A. K., Khodayari, A., Amiri, M., Boukherroub, R., & Szunerits, S. (2020). Aluminum based Metal-Organic Framework Integrated with Reduced Graphene Oxide for Improved Supercapacitive Performance. Electrochimica Acta, 353, 136609. https://doi.org/10.1016/j.electacta.2020.136609
Bansal, P., Mishra, D., Vijayakumar, A., & Chatterjee, S. (2023). Aluminium Terephthalate (Al-BDC) based Metal Organic Framework Decorated Carboxymethylated Filter Cloth For Defluoridation Application. Journal of Environmental Chemical Engineering, 11(3), 110233. https://doi.org/10.1016/j.jece.2023.110233.
Raju, U., Warkar, S. G., & Kumar, A. (2017). Green Synthesis of Multi Metal-Citrate Complexes and Their Characterization. Journal of Molecular Structure, 1133, 90–94. https://doi.org/10.1016/j.molstruc.2016.11.084
Horcajada, P., Gref, R., Baati, T., Allan, P. K., Maurin, G., Couvreur, P., Férey, G., Morris, R. E., & Serre, C. (2012). Metal–Organic Frameworks in Biomedicine. Chemical Reviews, 112(2), 1232–1268. https://doi.org/10.1021/cr200256v
Liu, J., Chen, L., Cui, H., Zhang, J., Zhang, L., & Su, C.-Y. (2014). Applications of Metal–Organic Frameworks in Heterogeneous Supramolecular Catalysis. Chemical Society Reviews, 43(16), 6011–6061. https://doi.org/10.1039/C4CS00094C
Cepeda, J., Pérez-Yáñez, S., Beobide, G., Castillo, O., Goikolea, E., Aguesse, F., Garrido, L., Luque, A., & Wright, P. A. (2016). Scandium/Alkaline Metal–Organic Frameworks: Adsorptive Properties and Ionic Conductivity. Chemistry of Materials, 28(8), 2519–2528. https://doi.org/10.1021/acs.chemmater.5b03458
Fujie, K., Ikeda, R., Otsubo, K., Yamada, T., & Kitagawa, H. (2015). Lithium Ion Diffusion in a Metal–Organic Framework Mediated by an Ionic Liquid. Chemistry of Materials, 27(21), 7355–7361. https://doi.org/10.1021/acs.chemmater.5b02986
Lee, H.-Y. (2011). A Study on the Synthesis of Aluminum Tartrate from Aluminum Chloride Solutions. Resources Recycling. 20(2), 54-59. https://doi.org/10.7844/kirr.2011.20.2.054
Greenstein, G. R. (2007). The Merck index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Reference Reviews, 21(6), 40-40. https://doi.org/10.1108/09504120710775534
Desroches, S., Daydé, S., & Berthon, G. (2000). Aluminum Speciation Studies in Biological Fluids. Journal of Inorganic Biochemistry, 81(4), 301–312. https://doi.org/10.1016/s0162-0134(00)00072-6
Khunur, M. M., & Prananto, Y. P. (2018). Synthesis and Structure of 2D Cobalt(II)-tartrate Hydrate Coordination Polymers Crystallised from Aqueous Solution. Bulletin of Chemical Reaction, Engineering, & Catalysis, 13(2), 213–219. https://doi.org/10.9767/bcrec.13.2.1342.213-219
Prananto, Y. P., Wibisono, R. D., Fadhilah, S. G., Tjahjanto, R., Darjito, & Salsabila, F. L. (2022). Crystallization of Mn(II) and Cd(II) Complexes in A Water-Methanol System: Tartrate vs Nicotinamide Ligand Selectivity. Acta Chimica Asiana, 5(1), 166–172. https://doi.org/10.29303/aca.v5i1.114
Rafika, A. H., Khunur, M. M., Tjahjanto, R. T. & Prananto, Y. P. (2022). Effect of Drying Temperature and Drying Time On The Crystallinity Degree of Zn(II)-tartrate Complex. Kuwait Journal of Chemistry, 50(4), 596–601. https://doi.org/10.1016/j.kjs.2023.03.007
Zokaee, Z., Mahmoodi, N. M., Rahimpour, M. R., & Shariati, A. (2022). Synthesis of visible light activated metal-organic framework coated on titania nanocomposite (MIL-53(Al)@TiO2) and dye photodegradation. Journal of Solid State Chemistry, 307, 122747. https://doi.org/10.1016/j.jssc.2021.122747
Author Biographies
Danar Purwonugroho, Brawijaya University
Department of Chemistry
M. Naufal Tsaqif Dzakwan, Brawijaya University
Department of Chemistry
Tutik Setianingsih, Brawijaya University
Department of Chemistry
Nidatul Syarifah, Brawijaya University
Department of Chemistry
License
Copyright (c) 2025 Yuniar Ponco Prananto, Danar Purwonugroho, M. Naufal Tsaqif Dzakwan, Tutik Setianingsih, Nidatul Syarifah

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish with ACA: Acta Chimica Asiana agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. This license allows authors to use all articles, data sets, graphics, and appendices in data mining applications, search engines, web sites, blogs, and other platforms by providing an appropriate reference. The journal allows the author(s) to hold the copyright without restrictions and will retain publishing rights without restrictions.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in ACA: Acta Chimica Asiana.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).





 Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia
Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia





