Increasing corrosion resistance of binary Al-Alloy through implanting with some transition elements and heteroatom organic compounds
Authors
Fatemeh MollaaminDOI:
10.29303/aca.v6i2.166Published:
2023-06-23Issue:
Vol. 6 No. 2 (2023)Keywords:
NHCs@TM–(Al–Mg), Sc; Ti, Cr; Ni, Cu; Z, Langmuir adsorption, N-heterocyclic carbenes, pyridine; alkylpyridines, CAM-B3LYP/EPR-III, LANL2DZ,6-31 G(d,p), DFT, ONIOMArticles
Downloads
How to Cite
Downloads
Metrics
Abstract
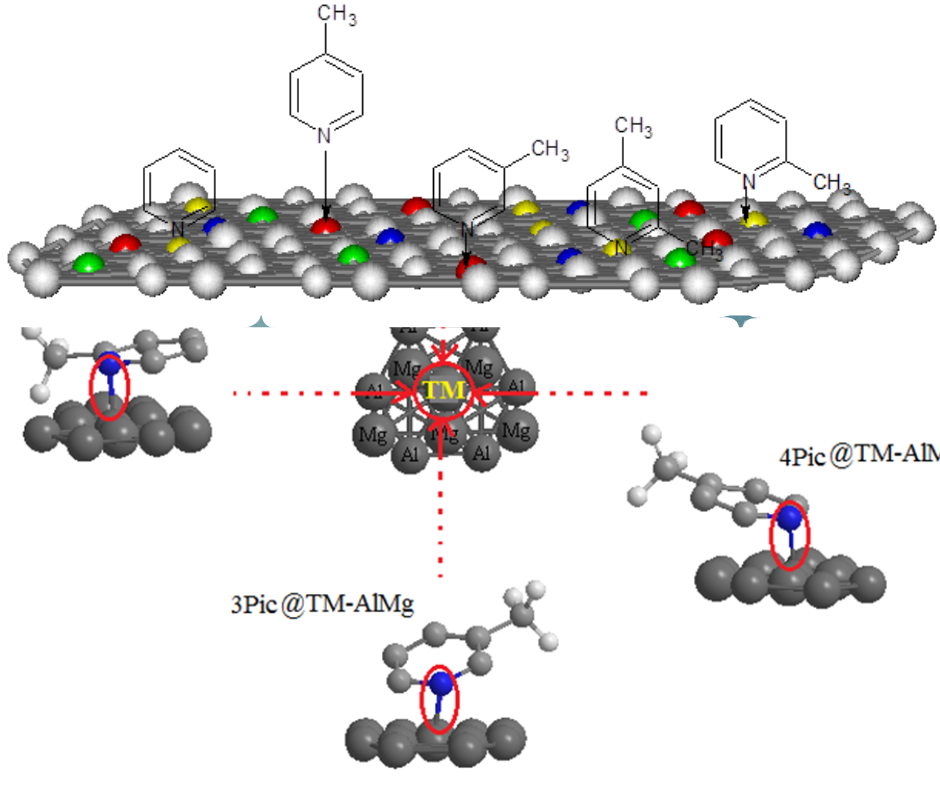
Decorating of Transition metals (TMs) on the "AlMg" nanoalloy has been studied on the basis of Langmuir adsorption applying "ONIOM" model with three levels of «high, medium and low» by using "LANL2DZ /6-31+G(d,p)/EPR-III", "semi-empirical" and "MM2" functions. The fluctuation of "NQR" has estimated the inhibiting role of pyridine and alkylpyridines containing 2-picoline (2Pic), 3-picoline (3Pic) ,4-picoline (4Pic), and 2,4-lutidine (24Lut) for (Sc, Ti, Cr, Ni, Cu, Zn)-doped AlMg alloy nanosheet due to "N" atom in the benzene cycle of heterocyclic carbenes being near the monolayer surface of ternary "TM–(Al–Mg)" (TM= Sc, Ti, Cr, Ni, Cu, Zn) nanoalloys. The "NMR" spectroscopy has remarked In fact, the NMR results of the adsorption of pyridine and alkylpyridines of 2Pic, 3Pic, 4Pic and 24Lut molecules represent spin polarization on the TM (Sc, Ti, Cr, Ni, Cu, Zn)-doped Al–Mg nanoalloy surfaces that these surfaces can be employed as the magnetic N-heterocyclic carbene sensors. In fact, "TM" sites in "TM–(Al–Mg)" nanoalloy surfaces have bigger interaction energy amount from "Van der Waals’ forces" with pyridine and its nitrogen heterocyclic family that might cause them large stable towards coating data on the nanosurface. It has been estimated that the criterion for choosing the surface linkage of "N" atom in pyridine and alkylpyridines in adsorption sites can be impacted by the existence of close atoms of aluminum and magnesium in the "TM–(AlMg)" surfaces. Moreover, "IR" spectroscopy has exhibited that (Sc, Ti, Cr, Ni, Cu, Zn)-doped AlMg alloy nanosheet with the fluctuation in the frequency of intra-atomic interaction leads us to the most considerable influence in the vicinage elements generated due to inter-atomic interaction. Comparison to amounts versus dipole moment has illustrated a proper accord among measured parameters based on the rightness of the chosen isotherm for the adsorption steps of the formation of Py@Sc–(Al–Mg), Py@Ti–(Al–Mg), Py@Cr–(Al–Mg), Py@Ni–(Al–Mg), Py@Cu–(Al–Mg), and Py@Zn–(Al–Mg) complexes. Thus, the interval between nitrogen atom in pyridine during interaction with transition metals of "Sc, Ti, Cr, Ni, Cu, Zn" in "TM–(Al–Mg)" nanoalloys, (N→TM), has been estimated with relation coefficient of R² = 0.9284. Thus, the present has exhibit the influence of "TMs" doped on the "Al–Mg" surface for adsorption of N-heterocyclic carbenes of pyridine and alkylpyridines by using theoretical methods.
References
Ahmad, Z., 2006. Principles of Corrosion Engineering and Corrosion Control. Elsevier Science and Technology Books.
Wei, C. Electrochemical deposition of aluminum. Technol. Innov. Appl. 2019, 18, 80-81.
Yang, H.M.; Qiu, Z.X.; Zhang, G. Low Temperature Aluminum Electrolysis; Northeast University Press: Shenyang, China, 2009.
Lu, H.M.; Qiu, Z.X. Research progress of low temperature aluminum electrolysis. Light Metals 1997, 4, 24.
Machnikova E, Whitmire KH, Hackerman N. Corrosion inhibition of carbon steel in hydrochloric acid by furan derivatives. Electrochim. Acta, 2008; 53: 6024–6032. DOI:10.1016/j.electacta.2008.03.021.
Benabdellah M, Touzani R, Aouniti A, et alInhibitive action of some bipyrazolic compounds on the corrosion of steel in 1 M HCl: Part I: Electrochemical study. Mater. Chem. Phys., 2007; 105: 373–379. DOI:10.1016/j.matchemphys.2007.05.001.
Fiala,A, Chibani A, Darchen A, et al. Investigations of the inhibition of copper corrosion in nitric acid solutions by ketene dithioacetal derivatives. Appl. Surf. Sci., 2007; 253: 9347–9356. DOI:10.1016/j.apsusc.2007.05.066.
Prabhu RA, Shanbhag AV, Venkatesha TV. Influence of tramadol [2-[(dimethylamino) methyl]-1-(3-methoxyphenyl) cyclohexanol hydrate] on corrosion inhibition of mild steel in acidic media. J Appl Electrochem 2007; 37: 491–497. DOI: 10.1007/s10800-006-9280-2.
Tian, G.C.; Li, J.; Hua, Y.X. Application of ionic liquids in metallurgy of nonferrous metals. Chin. J. Process Eng. 2009, 9, 200.
Tian, G.C.; Li, J.; Hua, Y.X. Application of ionic liquids in hydrometallurgy of nonferrous metals. Trans. Nonferrous Met. Soc. China 2010, 20, 513.
Monajjemi, M.; Afsharnezhad, S.; Jaafari, M.R.; Mirdamadi, S.; Mollaamin, F.; Monajemi, H. Investigation of energy and NMR isotropic shift on the internal rotation Barrier of Θ4 dihedral angle of the DLPC: A GIAO study. Chemistry 2008, 17, 55-69.
Tian, G.C. Ionic liquids as green electrolytes for Aluminum and Aluminum-alloy production. Mater. Res. Found. 2019, 54, 249.
Zhong, X.W.; Xiong, T.; Lu, J.; Shi, Z.N. Advances of electro-deposition and aluminum refining of aluminum and aluminum alloy in ionic liquid electrolytes system. Nonferrous Met. Sci. Eng. 2014, 5, 44.
Zheng, Y.; Wang, Q.; Zheng, Y.J.; Lv, H.C. Advances in research and application of aluminum electrolysis in ionic liquid systems. Chin. J. Process Eng. 2015, 15, 713.
Fleury, V.; Kaufman, J.H.; Hibbert, D.B. Mechanism of a morphology transition in ramified electrochemical growth. Nature 1994, 367, 435.
Yue, G.; Lu, X.; Zhu, Y.; Zhang, X.; Zhang, S. Surface morphology, crystal structure and orientation of aluminum coatings electrodeposited on mild steel in ionic liquid. Chem. Eng. J. 2009, 147, 79.
Barth, J. V.; Brune, H.; Ertl, G.; Behm, R. J. Scanning tunneling microscopy observations on the reconstructed Au(111) surface: Atomic structure, long-range superstructure, rotational domains, and surface defects. Phys. Rev. B. 1990, 42, 9307-9318.
Esken, D.; Turner, S.; Lebedev, O. I.; van Tendeloo, G.; Fischer, R. A. Au@ ZIFs: stabilization and encapsulation of cavity-size matching gold clusters inside functionalized zeolite imidazolate frameworks, ZIFs. Chem. Mater. 2010, 22, 6393-6401.
Mollaamin, F.; Monajjemi, M. Fractal Dimension on Carbon Nanotube-Polymer Composite Materials Using Percolation Theory. Journal of Computational and Theoretical Nanoscience 2012, 9, 597-601, https://doi.org/10.1166/jctn.2012.2067.
Liu, B.; Smit, B. Molecular Simulation Studies of Separation of CO2/N2, CO2/CH4, and CH4/N2 by ZIFs. J. Phys. Chem. C, 2010, 114, 8515-8522.
Keskin, S. Atomistic Simulations for Adsorption, Diffusion, and Separation of Gas Mixtures in Zeolite Imidazolate Frameworks. J. Phys. Chem. C, 2010, 115, 800-807.
Tran, U. P. N.; Le, K. K. A.; Phan, N. T. S. Expanding applications of metal- organic frameworks: zeolite imidazolate framework ZIF-8 as an efficient heterogeneous catalyst for the knoevenagel reaction. ACS Catal., 2011, 1, 120-127.
VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comp. Phys. Comm., 2005, 2, 103-128.
Phan, A.; Doonan, C. J.; Uribe-Romo, F. J.; Knobler, C. B.; O’keeffe, M.; Yaghi, O. M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res., 2010, 43, 58-67.
Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. B, 1964, 136, B864-B871.
Kohn, W.; Sham, L. J. Self-Consistent Equations Including Exchange and Correlation Effects.Phys. Rev., 1965, 140, A1133- A1138.
Lippert, G.; Hutter, J.; Parrinello, M. A hybrid Gaussian and plane wave density functional scheme. Mol. Phys., 1997, 92, 477-487.
Hartwigsen, C.; Goedecker, S.; Hutter, J. Relativistic separable dual-space Gaussian pseudopotentials from H to Rn. Phys. Rev. B, 1998, 58, 3641-3662.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple.Phys. Rev. Lett., 1996, 77, 3865-3868.
VandeVondele, J.; Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys., 2007, 127, 114105-1_114105-9.
Monajjemi, M.; Mahdavian, L.; Mollaamin, F. Characterization of nanocrystalline silicon germanium film and nanotube in adsorption gas by Monte Carlo and Langevin dynamic simulation. Bulletin of the Chemical Society of Ethiopia 2008, 22, 277-286, https://doi.org/10.4314/bcse.v22i2.61299.
Mavrikakis, M.; Stoltze, P.; Nørskov, J. K. Making gold less noble. Catal. Lett., 2000, 64, 101-106.
Dal Corso, A.; Ab initio phonon dispersions of transition and noble metals: effects of the exchange and correlation functional. J. Phys.; Condens. Matter, 2013, 25, 145401-145410.
Whitman, L. J.; Stroscio, J. A.; Dragoset, R. A.; Celotta, R. J. Geometric and electronic properties of Cs structures on III-V (110) surfaces: From 1D and 2D insulators to 3D metals. Phys. Rev. Lett., 1991, 66, 1338-1341.
Mollaamin, F.; Baei, M.T.; Monajjemi, M.; Zhiani, R.; Honarparvar, B. A DFT study of hydrogen chemisorption on V (100) surfaces. Russian Journal of Physical Chemistry A, Focus on Chemistry 2008, 82, 2354-2361, https://doi.org/10.1134/S0036024408130323.
Yildirim, H.; Greber, T.; Kara, A. Trends in Adsorption Characteristics of Benzene on Transition Metal Surfaces: Role of Surface Chemistry and van der Waals Interactions. J. Phys. Chem. C, 2013, 117, 20572-20583.
Monajjemi, M.; Baie, M.T.; Mollaamin, F. Interaction between threonine and cadmium cation in [Cd(Thr)] (n = 1-3) complexes: Density functional calculations , Russian Chemical Bulletin , 2010, 59. 886-889, https://doi.org/10.1007/s11172-010-0181-5.
Hoefling, M.; Iori, F.; Corni, S.; Gottschalk, K. E. The Conformations of Amino Acids on a Gold(111) Surface. ChemPhysChem., 2010, 11, 1763-1767.
Bakhshi, K.; Mollaamin, F.; Monajjemi, M. Exchange and Correlation Effect of Hydrogen Chemisorption on Nano V(100) Surface: A DFT Study by Generalized Gradient Approximation (GGA). Journal of Computational and Theoretical Nanoscience 2011, 8, 763-768, https://doi.org/10.1166/jctn.2011.1750.
Valencia, H.; Kohyama, M.; Tanaka, S.; Matsumoto, H. Ab initio study of EMIM-BF4 crystal interaction with a Li (100) surface as a model for ionic liquid/Li interfaces in Li-ion batteries. J. Chem. Phys. 2009, 131, 244705.
Clarke-Hannaford, J.; Breedon, M.; Best, A.S.; Spencer, M.J. The interaction of ethylammoniumtetrafluoroborate [EtNH3+][BF4−] ionic liquid on the Li (001) surface, towards understanding early SEI formation on Li metal. Phys. Chem. Chem. Phys. 2019, 21, 10028–10037.
Zhang, Q.Q. Study on Electrodeposition of Aluminum and Aluminum Alloy in Ionic Liquid; University of Chinese Academy of Sciences: Beijing, China, 2014.
Mollaamin, F.; Monajjemi, M. Harmonic Linear Combination and Normal Mode Analysis of Semiconductor Nanotubes Vibrations. J. Comput. Theor. Nanosci 2015, 12, 1030-1039. doi:10.1166/jctn.2015.3846.
Ali SA, Mazumder MAJ, Nazal MK, Al-Muallem HA. 2020 Assembly of succinic acid and isoxazolidine motifs in a single entity to mitigate CO2 corrosion of mild steel in saline media. Arab. J. Chem. 13, 242–257. (doi:10. 1016/j.arabjc.2017.04.005)
Amar H, Benzakour J, Derja A, Villemin D, Moreau B. 2003 A corrosion inhibition study of iron by phosphonic acids in sodium chloride solution. J. Electroanal. Chem. 558, 131–139. (doi:10.1016/S0022-0728(03)00388-7)
El-Sayed MS. 2011 Corrosion and corrosion inhibition of aluminum in Arabian Gulf seawater and sodium chloride solutions by 3-amino-5- mercapto-1,2,4-triazole. Int. J. Electrochem. Sci. 6, 1479–1492.
Sherif ES. 2014 A comparative study on the electrochemical corrosion behavior of iron and X-65 steel in 4.0 wt % sodium chloride solution after different exposure intervals. Molecules 19, 9962–9974. (doi:10.3390/ molecules19079962)
Yang D, Zhang M, Zheng J, Castaneda H. 2015 Corrosion inhibition of mild steel by an imidazolium ionic liquid compound: the effect of pH and surface pre-corrosion. RSC Adv. 5, 95 160–95 170. (doi:10.1039/ C5RA14556B).
Finšgar, M.; Milošev I. (11 March 2010). "Inhibition of copper corrosion by 1,2,3-benzotriazole: A review". Corrosion Science. 52 (9): 2737–2749. doi:10.1016/j.corsci.2010.05.002
Shen, A. Y.; Wu, S. N.; Chiu, C. T. (1999). "Synthesis and Cytotoxicity Evaluation of some 8-Hydroxyquinoline Derivatives". Journal of Pharmacy and Pharmacology. 51 (5): 543–548. doi:10.1211/0022357991772826.
CABASSI, PAJ; et al. (November–December 1983). "The improved flotation of gold from the residues of Orange Free State ores" (PDF). Journal of the South African Institute of Mining and Metallurgy. 83 (11): 270–276.
Monajjemi M, Mollaamin F, Gholami MR, et al. Quantum Chemical Parameters of Some Organic Corrosion Inhibitors, Pyridine, 2-Picoline 4-Picoline and 2, 4- Lutidine, Adsorption at Aluminum Surface in Hydrocholoric and Nitric Acids and Comparison Between Two Acidic Media. Main Group Met. Chem. 2003; 26: 349-362. https://doi.org/10.1515/MGMC.2003.26.6.349.
Mollaamin, F., Shahriari, S., Monajjemi, M. et al. Nanocluster of Aluminum Lattice via Organic Inhibitors Coating: A Study of Freundlich Adsorption. J Clust Sci 34, 1547–1562 (2023). https://doi.org/10.1007/s10876-022-02335-1
Mollaamin, F., Monajjemi, M. Corrosion Inhibiting by Some Organic Heterocyclic Inhibitors Through Langmuir Adsorption Mechanism on the Al-X (X = Mg/Ga/Si) Alloy Surface: A Study of Quantum Three-Layer Method of CAM-DFT/ONIOM. J Bio Tribo Corros 9, 33 (2023). https://doi.org/10.1007/s40735-023-00751-y.
Mollaamin F and Monajjemi M. Molecular modelling framework of metal-organic clusters for conserving surfaces: Langmuir sorption through the TD-DFT/ONIOM approach. MOLECULAR SIMULATION 2023; 49(4): 365–376. https://doi.org/10.1080/08927022.2022.2159996.
Mollaamin, F., Monajjemi, M. In Silico-DFT Investigation of Nanocluster Alloys of Al-(Mg, Ge, Sn) Coated by Nitrogen Heterocyclic Carbenes as Corrosion Inhibitors. J Clust Sci (2023). https://doi.org/10.1007/s10876-023-02436-5.
Zupanič, F.; Žist, S.; Albu, M.; Letofsky-Papst, I.; Burja, J.; Vončina, M.; Bončina, T. Dispersoids in Al-Mg-Si Alloy AA 6086 Modified by Sc and Y. Materials 2023, 16, 2949. https://doi.org/10.3390/ma16082949.
Li, S.; Dong, H.; Wang, X.; Liu, Z. Quenching Sensitivity of Al-Zn-Mg Alloy after Non-Isothermal Heat Treatment. Materials 2019, 12, 1595. https://doi.org/10.3390/ma12101595.
Wang, Z.; Zhang, P.; Zhao, X.; Rao, S. The Corrosion Behavior of Al-Cu-Li Alloy in NaCl Solution. Coatings 2022, 12, 1899. https://doi.org/10.3390/coatings12121899.
Svensson,M.; Humbel,S.; Froese,R.D.J.; Matsubara,T.; Sieber,S.; and Morokuma,K. ONIOM: A Multilayered Integrated MO + MM Method for Geometry Optimizations and Single Point Energy Predictions. A Test for Diels−Alder Reactions and Pt(P(t-Bu)3)2 + H2 Oxidative Addition. J. Phys. Chem. 1996, 100(50) , 19357–19363. https://doi.org/10.1021/jp962071j.
Ming-Dao,L., Lu-An, Y.,Qing-Yu, W., Xiao-Ci ,Y., Xiao-Dong, Y.,Jin-Yun, Z.,J.of Chinese Society of Corrosion and Protection.1996;16:3:195.
Sokkar, P.; Boulanger, E.; Thiel, W.; Sanchez-Garcia, E. J. Chem.
Theory Comput. 2015, DOI: 10.1021/ct500956u.
Felix Brandt, Christoph R. Jacob. Systematic QM Region Construction in QM/MM Calculations Based on Uncertainty Quantification. Journal of Chemical Theory and Computation 2022, 18 (4) , 2584-2596. https://doi.org/10.1021/acs.jctc.1c01093.
Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979.
Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865.
Ziesche, P.; Kurth, S.; Perdew, J.P. Density functionals from LDA to GGA. Comput. Mater. Sci. 1998, 11, 122–127.
Arrigoni, M.; Madsen, G.K.H. Comparing the performance of LDA and GGA functionals in predicting the T lattice thermal conductivity of III-V semiconductor materials in the zincblende structure: The cases of AlAs and BAs. Comput. Mater. Sci. 2019, 156, 354–360.
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652.
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789.
K. Kim; K. D. Jordan (1994). "Comparison of Density Functional and MP2 Calculations on the Water Monomer and Dimer". J. Phys. Chem. 98 (40): 10089–10094. doi:10.1021/j100091a024.
P. J. Stephens; F. J. Devlin; C. F. Chabalowski; M. J. Frisch (1994). "Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields". J. Phys. Chem. 98 (45): 11623–11627. doi:10.1021/j100096a001.
C. J. Cramer (2004). "Essentials of Computational Chemistry: Theories and Models, 2nd Edition Wiley". Wiley.com. Retrieved 2021-06-24.
S. H. Vosko; L. Wilk; M. Nusair (1980). "Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis". Can. J. Phys. 58 (8): 1200–1211. Bibcode:1980CaJPh..58.1200V. doi:10.1139/p80-159.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016.
GaussView, Version 6.06.16, Dennington, Roy; Keith, Todd A.; Millam, John M. Semichem Inc., Shawnee Mission, KS, 2016.
Sohail, U.; Ullah, F.; Binti Zainal Arfan, N.H.; Abdul Hamid, M.H.S.; Mahmood, T.; Sheikh, N.S.; Ayub, K. Transition Metal Sensing with Nitrogenated Holey Graphene: A First-Principles Investigation. Molecules 2023, 28, 4060. https://doi.org/10.3390/molecules28104060.
Smith, J. A. S. (January 1971). "Nuclear Quadrupole Resonance Spectroscopy". Journal of Chemical Education. 48: 39–41.
Appendix K: Nuclear quadrupole resonance, by Allen N. Garroway, Naval Research Laboratory. In Jacqueline MacDonald, J. R. Lockwood: Alternatives for Landmine Detection. Report MR-1608, Rand Corporation, 2003.
O. Kh. Poleshchuck, E.L. Kalinna, J. N. Latosinska, J. Koput, Journal of Molecular Structure (Theochem) 547 (2001) 233 – 243.
Young, Hugh A.; Freedman, Roger D. (2012). Sears and Zemansky's University Physics with Modern Physics (13th ed.). Boston: Addison-Wesley. p. 754.
License
Copyright (c) 2023 Fatemeh Mollaamin

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish with ACA: Acta Chimica Asiana agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. This license allows authors to use all articles, data sets, graphics, and appendices in data mining applications, search engines, web sites, blogs, and other platforms by providing an appropriate reference. The journal allows the author(s) to hold the copyright without restrictions and will retain publishing rights without restrictions.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in ACA: Acta Chimica Asiana.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).





 Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia
Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia





