Eco-friendly designing of zinc oxide nanoparticle as a potential semiconducting device for H2O-capture: a density functional theory study
Authors
Fatemeh MollaaminDOI:
10.29303/aca.v8i1.233Published:
2025-05-31Issue:
Vol. 8 No. 1 (2025)Keywords:
ZnO nanocluster, semiconductor, H/OH, adsorption, first principlesArticles
Downloads
How to Cite
Downloads
Metrics
Abstract
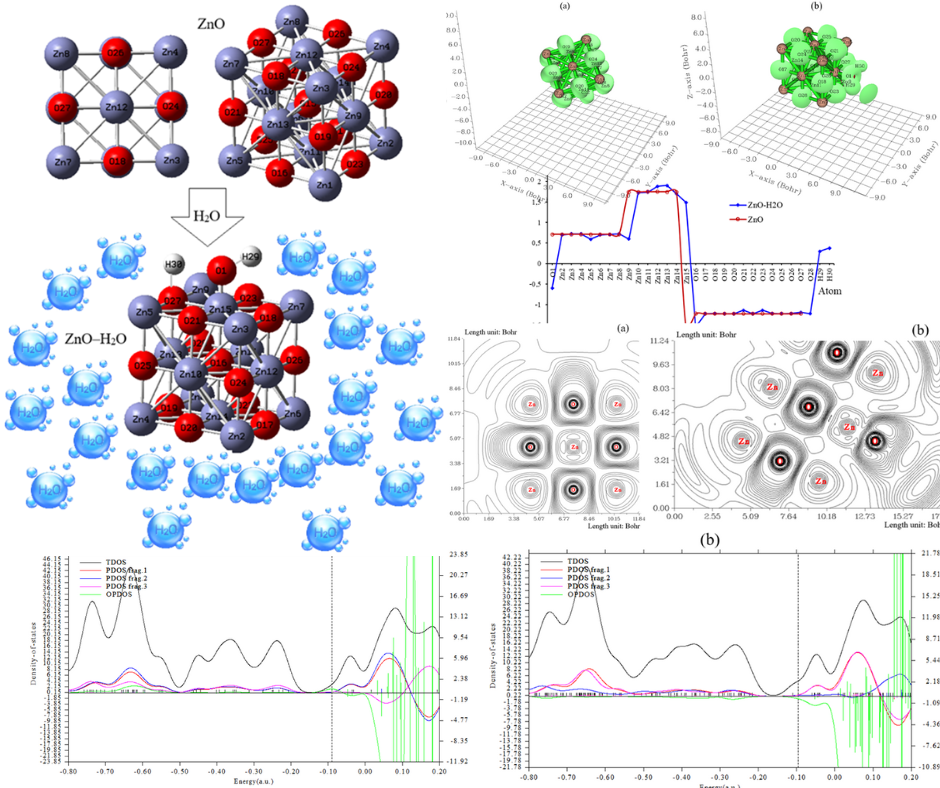
We employ first-principles calculations to investigate the structural stability and electronic properties of zinc oxide (ZnO) nanocluster adsorbed with H2O molecule. A comprehensive investigation on H2O grabbing by ZnO nanocluster was carried out using DFT computations at the CAM–B3LYP–D3/6–311+G (d, p) level of theory. The hypothesis of the energy adsorption phenomenon was confirmed by density distributions of CDD, TDOS/PDOS/OPDOS, and ELF for ZnO and ZnO–H2O. A vaster jointed area engaged by an isosurface map for H/OH adsorption on ZnO surface towards formation of ZnO–H2O complex due to labeling atoms of O1, Zn15, O27, H29, H30. Therefore, it can be considered that zinc in the functionalized ZnO might have more impressive sensitivity for accepting the electrons in the process of H/OH adsorption. It is considerable that when all surface atoms of ZnO are coated by OH and H groups, the semiconducting behavior is recovered. Our results open up the possibility of tailoring the electronic properties by controlling the surface adsorption sites. The nanoclusters of bare ZnO and ZnO–H2O can be defined by ELF graphs owing to exploring their delocalization/localization characterizations of electrons and chemical bonds. The results indicate that the stability and the optical gap are related to the sizes and symmetries of the clusters. Further, it is shown that the structures have much greater impact on the optical gap, there is the dipole-forbidden transition in the optical gap for high symmetric structures.
References
S.Z. Zainabidinov, A.Y. Boboev, M.B. Rasulova, N.Y. Yunusaliyev. X-ray diffraction analysis, optical characteristics and electro-physical properties of the N-ZnO/P-NiO structure grown by the spray pyrolysis method. New Materials, Compounds and Applications. 8(3), 2024, 411-421. https://doi.org/10.62476/nmca83411
S. Parmonov, K. Sharipov, D. Mirzavaliyev, F. Makhmudova, N. Askarova, R. Toshkodirova, N. Abdullaeva, M. Rifky, J.M. Harris, A. Kambarov. The technology of obtaining powders used in the production of solid alloys. New Materials, Compounds and Applications. 8(3), 2024, 450-458. https://doi.org/10.62476/nmca83450
Monajjemi, M., Mohammadi, S., Shahriari, S. et al. Experimental and Theoretical Studies of ZnO Nanotubes: an Approach to Chemical Physics Characterization of ZnONTs, Including Morphology, Piezoelectric, and Density of States. Russ. J. Phys. Chem. B 18, 308–324 (2024). https://doi.org/10.1134/S1990793124010342
Vasin, A.A., Dobryakov, A.L., Kochev, S.Y. et al. Relaxation of Multiple Excitons in ZnCdS and ZnCdS/ZnS Alloy Quantum Dots. Russ. J. Phys. Chem. B 18, 1646–1650 (2024). https://doi.org/10.1134/S1990793124701380
Olkhovskaya, I.P., Krokhmal, I.I. & Glushchenko, N.N. Improvement of the Morphophysiological Parameters of Pepper after the Presowing Treatment of Seeds with Zinc Nanoparticles. Russ. J. Phys. Chem. B 18, 527–532 (2024). https://doi.org/10.1134/S1990793124020295
I.I. Abbasov, M.A. Musayev, C.I. Huseynov, Q.Y. Eyyubov, N.N. Hasimova, A.J. Mammadova, A.A. Hadieva, Y.I. Aliyev, N.A. Qasumov, R.Sh. Rahimov. A study of impurity defect photoluminescence in ZnSe:Cr and ZnSe:Fe in the near infrared at room temperature. Advanced Physical Research. 5(3), 2023, 192-199
Zhang, X.Q., Zhang, B. Effect of Pressure on the Structural, Mechanical, and Electronic Properties of Monoclinic ZnWO4. Russ. J. Phys. Chem. B 17, 1049–1056 (2023). https://doi.org/10.1134/S1990793123050135
Ikim, M.I., Spiridonova, E.Y., Gromov, V.F. et al. Effect of the Formation Method of ZnO–In2O3 Composites on Their Structural Characteristics and Conductivity. Russ. J. Phys. Chem. B 18, 283–288 (2024). https://doi.org/10.1134/S199079312401010X
Zverev, A.S., Zvekov, A.A., Pugachev, V.M. et al. Controlling the Sensitivity of Pentaerythritol Tetranitrate to Visible Laser Radiation by the Addition of ZnO:Ag Nanopowder. Russ. J. Phys. Chem. B 17, 1135–1142 (2023). https://doi.org/10.1134/S1990793123050147
Yuan, X., Tan, X. & Liu, B. Structural, Mechanical, Electronic and Optical Properties of Spinel ZnAl2O4 Underpressure from First-Principles Calculations. Russ. J. Phys. Chem. B 17, 886–895 (2023). https://doi.org/10.1134/S1990793123040322
Martin, O.; González, V.; Tirado, M.; Comedi, D. Effects of methanol on morphology and photoluminescence in solvothermal grown ZnO powders and ZnO on Si. Mater. Lett. 2019, 251, 41–44.
Xiong, H.M.; Liu, D.P.; Xia, Y.Y.; Chen, J.S. Polyether-grafted ZnO Nanoparticles with Tunable and Stable Photoluminescence at Room Temperature. Chem. Mater. 2005, 17, 3062–3064.
Agarwal, D.C.; Singh, U.B.; Gupta, S.; Singhal, R.; Kulriya, P.K.; Singh, F.; Tripathi, A.; Singh, J.; Joshi, U.S.; Avasthi, D.K. Enhanced room temperature ferromagnetism and green photoluminescence in Cu doped ZnO thin film synthesised by neutral beam sputtering. Sci. Rep. 2019, 9, 6675.
Wolska, E.; Kaszewski, J.; Kiełbik, P.; Grzyb, J.; Godlewski, M.M.; Godlewski, M. Rare earth activated ZnO nanoparticles as biomarkers. Opt. Mater. 2014, 36, 1655–1659
Lim, J.H.; Kang, C.K.; Kim, K.K.; Park, I.K.; Hwang, D.K.; Park, S.J. UV Electroluminescence Emission from ZnO Light-Emitting Diodes Grown by High-Temperature Radiofrequency Sputtering. Adv. Mater. 2006, 18, 2720–2724.
Tolubayeva, D.B.; Gritsenko, L.V.; Kedruk, Y.Y.; Aitzhanov, M.B.; Nemkayeva, R.R.; Abdullin, K.A. Effect of Hydrogen Plasma Treatment on the Sensitivity of ZnO Based Electrochemical Non-Enzymatic Biosensor. Biosensors 2023, 13, 793. https://doi.org/10.3390/bios13080793
Liu, H.; Zhang, Y.; Zhang, H.; Wang, L.; Wang, T.; Han, Z.; Wu, L.; Liu, G. Effect of plasma vitamin C levels on Parkinson’s disease and age at onset: A Mendelian randomization study. J. Transl. Med. 2021, 19, 221.
Abdullin, K.A.; Gabdullin, M.T.; Gritsenko, L.V.; Ismailov, D.V.; Kalkozova, Z.K.; Kumekov, S.E.; Mukash, Z.O.; Sazonov, A.Y.; Terukov, E.I. Electrical, Optical, and Photoluminescence Properties of ZnO Films Subjected to Thermal Annealing and Treatment in Hydrogen Plasma. Semiconductors 2016, 50, 1010–1014.
Chang, S.-C.; Hu, J.-C.; Chan, H.-T.; Hsiao, C.-A. Influence of Processing Time in Hydrogen Plasma to Prepare Gallium and Aluminum Codoped Zinc Oxide Films for Low-Emissivity Glass. Coatings 2022, 12, 945.
Chang, S.-C.; Li, T.-H.; Chan, H.-T. Hydrogen Plasma Annealed Titanium Dioxide Oxide/Aluminum-doped Zinc Oxide Films Applied in Low Emissivity. Glass. Int. J. Electrochem. Sci. 2021, 16, 210817.
Zhang, C.; Cao, Z.; Zhang, G.; Yan, Y.; Yang, X.; Chang, J.; Song, Y.; Jia, Y.; Pan, P.; Mi, W.; et al. An electrochemical sensor based on plasma-treated zinc oxide nanoflowers for the simultaneous detection of dopamine and diclofenac sodium. Microchem. J. 2020, 158, 105237.
Srijita NundyAritra Ghosh*Tapas K. Mallick, Hydrophilic and Superhydrophilic Self-Cleaning Coatings by Morphologically Varying ZnO Microstructures for Photovoltaic and Glazing Applications. ACS Omega 2020, 5, 2, 1033–1039. https://doi.org/10.1021/acsomega.9b02758
David Raymand, Adri C.T. van Duin, Daniel Spångberg, William A. Goddard III, Kersti Hermansson, Water adsorption on stepped ZnO surfaces from MD simulation, Surface Science, 604(9–10), 741–752 (2010). https://doi.org/10.1016/j.susc.2009.12.012
Mollaamin, F., Monajjemi, M. Designing novel nanomaterials for Li-ion batteries: A physico-chemical study through hydrogen-powered horizons. New Materials, Compounds and Applications, 8(3), 303-323 (2024). https://doi.org/10.62476/nmca83303
F. Mollaamin, M. Monajjemi, Application of nanoscale boron nitride for encapsulation of noxious transition metals (Cr, Mn, Fe, Zn, W, Cd) in soil: Physico-chemical characterization using DFT modeling. Advanced Physical Research. 7(1), 2025, 5-28. https://doi.org/10.62476/apr.7105
Fatemeh Mollaamin, Majid Monajjemi, Determination of GaN nanosensor for scavenging of toxic heavy metal ions (Mn2+, Zn2+, Ag+, Au3+, Al3+, Sn2+) from water: Application of green sustainable materials by molecular modeling approach. Computational and Theoretical Chemistry. 1237, 114646, 2024. https://doi.org/10.1016/j.comptc.2024.114646
Mollaamin, F., Monajjemi, M. Structural, Electromagnetic and Thermodynamic Analysis of Ion Pollutants Adsorption in Water by Gallium Nitride Nanomaterial: a Green Chemistry Application. Russ. J. Phys. Chem. B 18, 533–548 (2024). https://doi.org/10.1134/S199079312402012X
Fatemeh Mollaamin, Majid Monajjemi, Trapping of toxic heavy metals from water by GN–nanocage: Application of nanomaterials for contaminant removal technique. Journal of Molecular Structure. 1300, 137214, 2024, https://doi.org/10.1016/j.molstruc.2023.137214.
Mollaamin, F., Monajjemi, M. Tailoring and functionalizing the graphitic-like GaN and GaP nanostructures as selective sensors for NO, NO2, and NH3 adsorbing: a DFT study. J Mol Model 29, 170 (2023). https://doi.org/10.1007/s00894-023-05567-8
F. Mollaamin, M. Monajjemi, In Silico-DFT Investigation of Nanocluster Alloys of Al-(Mg, Ge, Sn) Coated by Nitrogen Heterocyclic Carbenes as Corrosion Inhibitors, J Clust Sci, 34 (6), 2901–2918, 2023. https://doi.org/10.1007/s10876-023-02436-5
Mollaamin, F.; Monajjemi, M. Doping of Graphene Nanostructure with Iron, Nickel and Zinc as Selective Detector for the Toxic Gas Removal: A Density Functional Theory Study. C 2023, 9, 20. https://doi.org/10.3390/c9010020
F. Mollaamin, M. Monajjemi, Transition metal (X = Mn, Fe, Co, Ni, Cu, Zn)-doped graphene as gas sensor for CO2 and NO2 detection: A molecular modeling framework by DFT perspective, J. Mol. Model., 29(4), 119, 2023. https://doi.org/10.1007/s00894-023-05526-3
F. Mollaamin, S. Shahriari, M. Monajjemi, Z. Khalaj, Nanocluster of Aluminum Lattice via Organic Inhibitors Coating: A Study of Freundlich Adsorption, J. Clust. Sci., 34(3), 1547–1562, 2023. https://doi.org/10.1007/s10876-022-02335-1
Mollaamin, F., Monajjemi, M. In Situ Ti-Embedded SiC as Chemiresistive Nanosensor for Safety Monitoring of CO, CO2, NO, NO2: Molecular Modelling by Conceptual Density Functional Theory. Russ. J. Phys. Chem. B 18, 49–66 (2024). https://doi.org/10.1134/S1990793124010159
Mollaamin, F., Monajjemi,M., Adsorption ability of Ga5N10 nanomaterial for removing metal ions contamination from drinking water by DFT, Int J Quantum Chem, 2024, 124, e27348. https://doi.org/10.1002/qua.27348.
Mollaamin F and Monajjemi M. Molecular modelling framework of metal-organic clusters for conserving surfaces: Langmuir sorption through the TD-DFT/ONIOM approach. Molecular Simulation 2023; 49(4): 365–376. https://doi.org/10.1080/08927022.2022.2159996.
Mollaamin, F. Competitive Intracellular Hydrogen-Nanocarrier Among Aluminum, Carbon, or Silicon Implantation: a Novel Technology of Eco-Friendly Energy Storage using Research Density Functional Theory. Russ. J. Phys. Chem. B 18, 805–820 (2024). https://doi.org/10.1134/S1990793124700131
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; et al. Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016.
GaussView, Version 6.06.16, Dennington, Roy; Keith, Todd A.; Millam, John M. Semichem Inc., Shawnee Mission, KS, 2016.
Zihan Xu, Chenglong Qin, Yushu Yu, Gang Jiang, Liang Zhao, First-principles study of adsorption, dissociation, and diffusion of hydrogen on α-U (110) surface. AIP Advances 14, 055114 (2024). https://doi.org/10.1063/5.0208082
T. Lu & F. Chen, Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012). doi.org/10.1002/jcc.22885
T. Lu, A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 161, 082503 (2024). doi.org/10.1063/5.0216272
Mollaamin, F., Monajjemi, M. Perspective of Clean Energy-saving by Semiconducting Quantum Dot Nanomaterials through Photoelectric and Density of States Analysis. J Fluoresc (2025). https://doi.org/10.1007/s10895-025-04207-z
Steinmann,S.N. Mob, Y. Corminboeuf,C. How do electron localization functions describe π-electron delocalization? Phys. Chem. Chem. Phys. 13(2011)20584-20592. https://doi.org/10.1039/C1CP21055F
Tian, L. Feiwu, C. Xuebao, W. Meaning and Functional Form of the Electron Localization Function, Acta Phys. Chim. Sin. 27 (2011) 2786–2792. https://doi.org/10.3866/PKU.WHXB20112786
M.F. Thanoon, L.M. Al-Nema, Hardness, elastic modulus, water solubility and Fourier Transformation Infrared (FTIR) of the modified soft liner with two types of plasticizers. New Materials, Compounds and Applications. 8(2), 2024, 233-243 https://doi.org/10.62476/nmca82233
I. Mayer, Improved definition of bond orders for correlated wave functions. Chemical Physics Letters 544, 83-86 (2012). https://doi.org/10.1016/j.cplett.2012.07.0
Mollaamin, F. and Monajjemi, M. (2023), Graphene-based resistant sensor decorated with Mn, Co, Cu for nitric oxide detection: Langmuir adsorption & DFT method. Sensor Review. 43(4), 266-279. https://doi.org/10.1108/SR-03-2023-0040
Tian Lu and Feiwu Chen, Bond Order Analysis Based on the Laplacian of Electron Density in Fuzzy Overlap Space. J. Phys. Chem. A 2013, 117, 14, 3100–3108. https://doi.org/10.1021/jp4010345
S.H.M. Sheet, R.B. Mahmod, N.H.M. Saeed, S.M. Saied, Theoretical study for comparison of pKa of a number of Achiff bases by employing parameters derived from DFT and MP2 method. New Materials, Compounds and Applications. 8(1), 2024, 94-108. https://doi.org/10.62476/nmca8194
Juliusz Winiarski, Włodzimierz Tylus, Katarzyna Winiarska, Irena Szczygieł, Bogdan Szczygieł, XPS and FT-IR Characterization of Selected Synthetic Corrosion Products of Zinc Expected in Neutral Environment Containing Chloride Ions. HindawiJournal of Spectroscopy, 2018, 2079278. https://doi.org/10.1155/2018/2079278
License
Copyright (c) 2025 Fatemeh Mollaamin

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish with ACA: Acta Chimica Asiana agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. This license allows authors to use all articles, data sets, graphics, and appendices in data mining applications, search engines, web sites, blogs, and other platforms by providing an appropriate reference. The journal allows the author(s) to hold the copyright without restrictions and will retain publishing rights without restrictions.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in ACA: Acta Chimica Asiana.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).





 Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia
Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia





