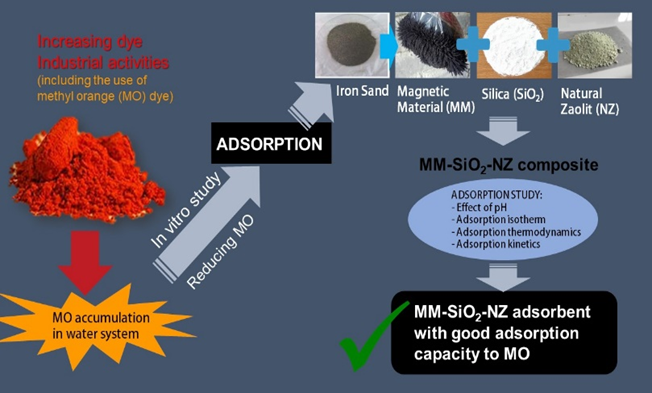
Thermodynamics and kinetic studies of methyl orange dye adsorption in magnetic material-silica-zeolite (MM-Sio2-NZ) composite
DOI:
10.29303/aca.v6i2.159Published:
2022-05-10Issue:
Vol. 6 No. 2 (2023)Keywords:
adsorption, methyl orange, magnetic material, silica, zeoliteArticles
Downloads
How to Cite
Armid, A., Fahmiati, F., Ritonga, H., Ismail, D. ., & Ramadhan, L. O. A. N. . (2022). Thermodynamics and kinetic studies of methyl orange dye adsorption in magnetic material-silica-zeolite (MM-Sio2-NZ) composite. Acta Chimica Asiana, 6(2), 301–308. https://doi.org/10.29303/aca.v6i2.159
Downloads
Download data is not yet available.
Metrics
Metrics Loading ...






 Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia
Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia





