Quantum Dynamic Approach of B2N(∓,0) and N2B (∓,0) Clusters Study: A Symmetry Breaking due to the Jahn-Teller Effect
Authors
Majid MonajjemiDOI:
10.29303/aca.v7i1.168Published:
2024-04-17Issue:
Vol. 7 No. 1 (2024)Keywords:
Boron nitride, 〖B_2 N〗^((∓,0)), 〖N_2 B〗^((∓,0)), Jahn-Teller, Symmetry BreakingArticles
Downloads
How to Cite
Downloads
Metrics
Abstract
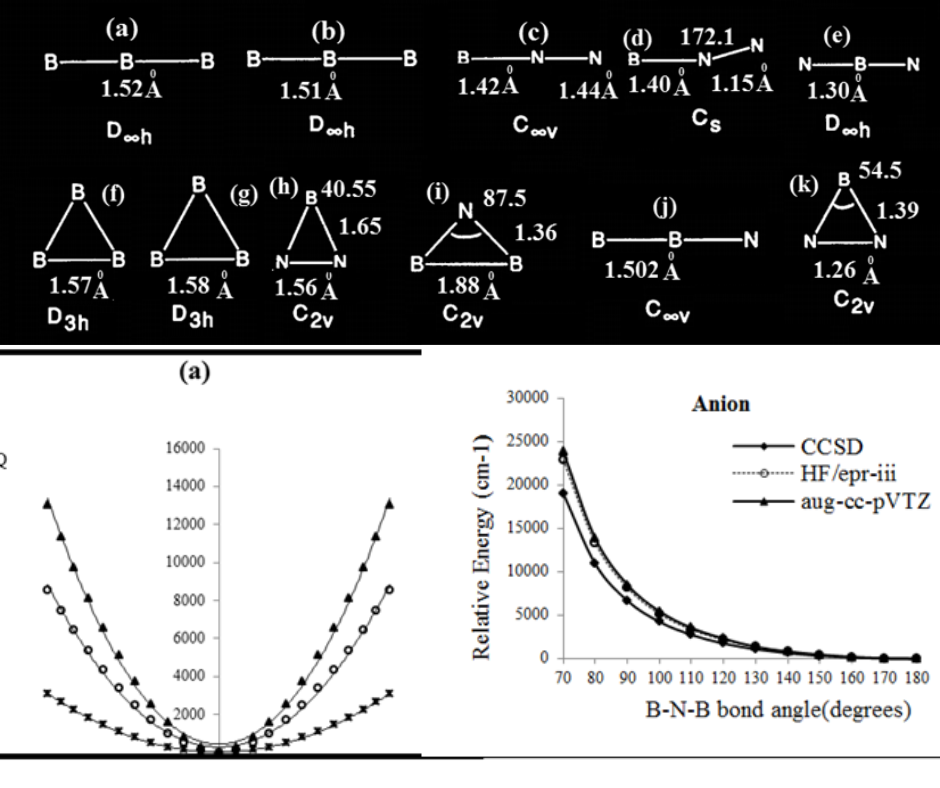
BN compounds play an important role in the preparation of hyper- thin films, that have received signifcant attention in products. In this article, we investigated the electronic structures of and. Triatomic NBN and BNB have recently been studied using various experimental and calculation approaches, and it is totally agreed that both of them are linear in their ground electronic step. The six ions including B2N (-), B2N (+), B2N (0) , BN2 (-), BN2 (+) and BN2 (0) have been studied and been also compared with one another in terms of several basis sets and predication of the symmetry breaking (SB) subject. Artifactual SB with the v3 vibration is occurred in the trial wave functions of coupled-cluster level, even when Brueckner orbitals of all nitrogen and oxygen atoms are used. In the of and molecules, the unpaired electrons are delocalized, while in the asymmetric, they are localized on either one of the B atoms or N atoms of and , respectively. Structures with (SB),, can be stronger by interaction to the . Hereby, the second-order Jahn-Teller effect allows the unpaired electron to localize on boron atom, rather than being delocalized. Finally, from a statistical thermodynamical analysis, we calculated the thermodynamically stabilities of those six ions.
References
J.M.L. Martin, J.-P. François, and R. Gijbels, Chem. Phys., 90, 6469 (1989). doi: org/ 10.1063/1.456313.
K.R.Asmis, T.R.Taylor, and D.M. Neumark, J. Chem. Phys., 111, 8838 (1999). doi: org/ 10.1063/1.480230.
J.M.L.Martin, J.-P.François, and R. Gijbels, Chem. Phys. Lett., 172, 354 (1990). doi: org/10.1016/s0009-2614(90)87126-c.
L.B.Knight, Jr.D.W.Hill, T.J.Kirk, and C.A. Arrington, J. Phys. Chem., 96, 5604 (1992). https://doi.org/10.1063/1.462703.
J. M. L.Martin, J.-P.François, and R. Gijbels, Chem. Phys. Lett. 193, 243 (1992). doi: org/10.1016/0009-2614(92)85662-T.
P. Hassanzadeh, and L. Andrews, J. Phys. Chem. 96, 9177 (1992). doi: org/10.1021/j100202a020.
L. Andrews, P. Hassanzadeh, T. R. Burkholder, and J. M. L. Martin, J.Chem. Phys. 98, 922 (1993). doi: org/10.1063/1.464256.
C. A. Thompson, and L. Andrews, J. Am. Chem. Soc. 117, 10125 (1995). doi: org/10.1021/ja00145a029.
X. Li, and J. Paldus, J. Chem. Phys. 126, 224304 (2007). doi: org/10.1063/1.2746027.
J.M.L. Martin, J. El-Yazal, Mol. Phys. 85,527 (1995). doi: org/10.1080/00268979500101281.
G.Meloni, M. Sai Baba, and A. Gingerich, J. Chem. Phys. 113, 8995 (2000).doi: org/10.1063/1.1319353.
W. R. M. Graham, and W. Weltner Jr., J. Chern. Phys. 65, 1516 (1976). doi: org/10.1063/1.433206.
M. Monajjemi, Chemical Physics. 425, 29 (2013). doi: org/10.1016/j.chemphys.2013.07.014.
M.Monajjemi, V.S.Lee, M.Khaleghian, B.Honarparvar, F. Mollaamin, J. Phys. Chem. C, 114, 15315 (2010). doi: org/10.1021/jp104274z.
M.Monajjemi, J.E. Boggs, J. Phys. Chem. A, 117, 1670 (2013). doi: org/10.1021/jp312073q.
M. Monajjemi, Struct. Chem. 23, 551 ( 2012). doi: org /10.1007 /s11224-011-9895-8.
L. Mahdavian, M. Monajjemi, Microelectronics journal, 41, 142 (2010). doi: org/10.1016/j.mejo.2010.01.011.
Löwdin, P.O. Rev. Mod. Phys. 35, 496 (1963). doi: org/10.1103/REVMODPHYS.35.724.
E. P. Wigner, Group Theory and its Application to the Quantum Mechanics of Atomic Spectra, Academic Press, New York, p. 259 (1959).
G. Seifert, B. Schwab, S. Becker, and H. J. Dietze, Int. J. Mass Spectr. Ion
Proc. 85, 327 (1988).
V. Barone, Recent Advances in Density functional methods, parts I, Ed. D.P.Chong , world scientific publ. Co, springer, (1996).
X.Blase, A.Rubio, S.G.Louie, and M.L. Cohen, Europhysics Letters (EPL), 28, 335 (1994). doi: org/10.1209/0295-5075/28/5/007.
X. Blase, J.C. Charlier, A.de.Vita, R. Car, Appl. Phys. Lett. 70, 197 (1997). doi: org/10.1063/1.118354.
W.Han, Y.Bando, K.Kurashima, and T. Sato, Appl. Phys. Lett. 73, 3085 (1998). doi: org/10.1063/1.122680.
T.E.H. Walker, W. G. Richards, J.chem.phys. 52, 1311 (1970). doi: org/10.1063/1.1673131.
S. Koseki, M.W. Schmidt, and M.S. Gordon, J.phys.chem. 96, 10768 (1992). doi: org/10.1021/j100205a033.
J.A. Pople, M. Head-Gordon, and K. Raghavachari, J.chem.phys., 87, 5968 (1987). https://doi.org/10.1063/1.453520.
R.F.W. Bader, Atoms in Molecule: A quantum Theory , Oxford Univ. press, Oxford, (1990).
B.H. Besler, K.M. Merz Jr., and P.A. Kollman, J. comp. Chem. 11, 431 (1990). doi: org/10.1002/jcc.540110404.
L.E. Chirlian, and M.M. Francl, J.comp.chem. 8, 894 (1987). doi: org/10.1002/jcc.540080616.
C.M. Breneman, and K.B. Wiberg, J. Comp.Chem. 11, 361 (1990). doi: org/10.1002/jcc.540110311.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, (2016).
G. Herzberg, Infrared and Raman Spectra ofPolyatomic Molecules (Van
Nostrand Reinhold, New York), p. 521 If. (1945).
Manual ofsymbols and Terminology for Physico-chemical Quantities and
Units (prepared by M. L. McGlashan, revision prepared by M. A. Paul,
second revision by D. H. Whilfen) , Pure Appl. Chern. 51,1 (1979).
S. Bagheri, M.Monajjemi, A. Ziglari , A. Taghvamanesh ,Russian Journal of Physical Chemistry B, 15, S140-S148 (2021), doi: org/10.1134/S1990793121090049.
M. A. Ashraf, Z. Liu & M. Najafi , Russian Journal of Physical Chemistry B, 14, 217-221 (2020), doi: org/10.1134/S1990793120020189.
A. Yu. Shaulov, L. V. Vladimirov, A. V. Grachev, V. M. Lalayan, E. M. Nechvolodova, R. A. Sakovich, V. K. Skachkova, E. V. Stegno, L. A. Tkachenko, S. A. Patlazhan & A. A. Berlin, Russ. J. Phys. Chem. B, 14, 183-189 (2020). doi:org/10.1134/S1990793120010157.
E.A. Lebedeva, S.A. Astaf’eva, T.S. Istomina, Russ. J. Phys. Chem. B, 16, 316-322 (2022). doi: org/10.1134/S1990793122010109.
A.G. Korotkikh, I.V. Sorokin, E.A. Selikhova, V. A. Arkhipov, Russ. J. Phys. Chem. B, 14, 592-600 (2020). doi :org/10.1134/S1990793120040089.
A.G. Korotkikh, I.V. Sorokin, V.A. Arkhipov, Russ. J. Phys. Chem. B, 16, 253-259 (2022). doi: org/10.1134/S1990793122020075.
Gerasimov, G. N. ; Gromov,V. F.; Ikim, M. I.; L. I. Trakhtenberg, Effect of Composition and Structure of Metal Oxide Composites Nanostructured on Their Conductive and Sensory Properties, Russ. J. Phys. Chem., 2021, 15, 1072–1083. https://doi.org/ 10.1134/ S199079 31 21310018.
Kablov, V.F., Strakhov, V.L., Kaledin, V.O. et al. Mathematical Modeling of the Physicochemical Properties of a Heat-Shielding Material from Highly Filled Elastomers. Russ. J. Phys. Chem. B 2021, 15, 880–887. https://doi.org/ 10.1134/ S199079 31210 50043.
Sarvendra Kumar, Surbhi & Yadav, M.K. Optimized Molecular Geometries, Internal Coordinates, Vibrational Analysis, Thermodynamic Properties, First Hyperpolarizability and HOMO–LUMO Analysis of Duroquinone Using Density Functional Theory and Hartree–Fock Method. Russ. J. Phys. Chem. B 2021, 15 (Suppl 1), S22–S31. https://doi.org/ 10.1134/ S1990793121090116.
Sakovich, R.A., Shaulov, A.Y., Nechvolodova, E.M. et al. Energy of Intramolecular Interactions and Structure of Metallophosphate Polycomplexes with Water Molecules and Nitrogen-Containing Compounds. Russ. J. Phys. Chem. B 2020, 14, 516–521. https://doi.org/10.1134/S1990793120030094.
License
Copyright (c) 2024 Majid Monajjemi

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish with ACA: Acta Chimica Asiana agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. This license allows authors to use all articles, data sets, graphics, and appendices in data mining applications, search engines, web sites, blogs, and other platforms by providing an appropriate reference. The journal allows the author(s) to hold the copyright without restrictions and will retain publishing rights without restrictions.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in ACA: Acta Chimica Asiana.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).





 Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia
Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia





