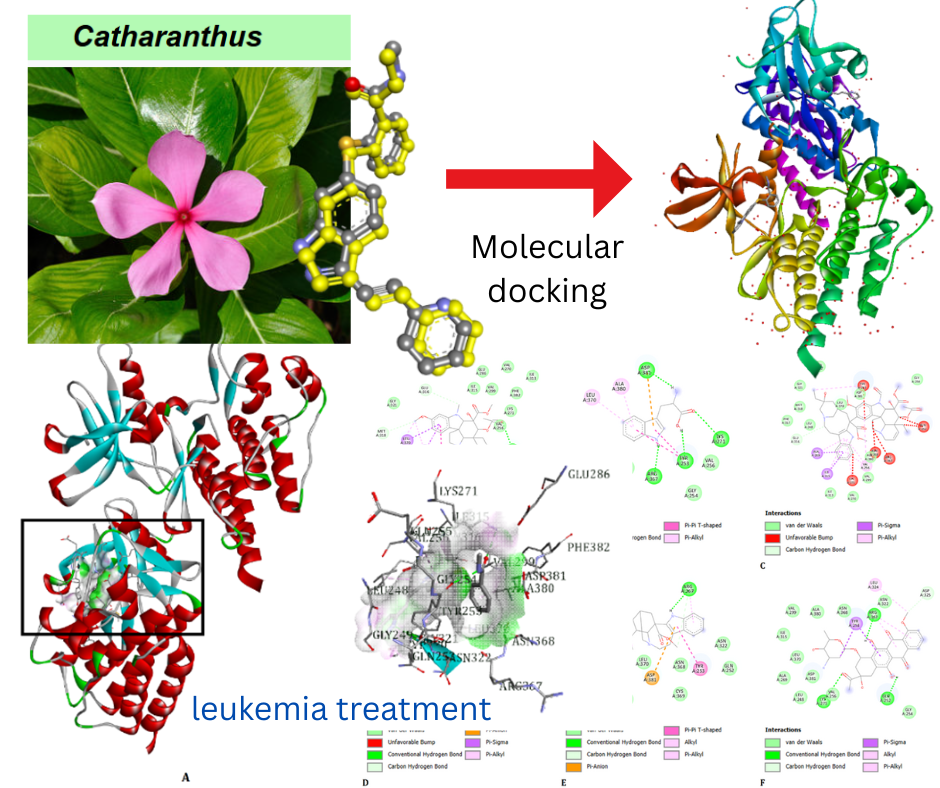
Molecular docking study of catharanthus roseus compounds as potential ABL1 inhibitors for leukemia treatment
Authors
Muhammad Farid , Shalahuddin Al Madury , Ahmad Suriyadi Muslim , Zakiyyah Qurrotul ‘AiniDOI:
10.29303/aca.v8i1.235Published:
2025-05-31Issue:
Vol. 8 No. 1 (2025)Keywords:
Leukemia, ABL1, Catharanthus roseus, molecular docking, ADMETArticles
Downloads
How to Cite
Downloads
Metrics
Abstract
Leukemia is one type of cancer with a high mortality rate, caused by the proliferation of abnormal white blood cells that disrupt hematopoiesis function. Conventional therapies, such as chemotherapy and targeted therapy, often face challenges in the form of side effects and drug resistance. This study aims to evaluate the potential of Catharanthus roseus as a leukemia therapeutic agent through in silico. The docking process uses autodock, and ADMET prediction uses SwissADME and PKCMS. The study used the ABL1 protein (PDB ID: 4TWP) as a target with active compounds of Catharanthus roseus. The validation process of the docking method showed an RMSD value of 0.705 Å, indicating that the method used was valid. The results of the docking simulation showed that vindoline had the best affinity after native ligands with a binding energy of -8.64 kcal/mol, followed by catharanthine -6.16 kcal/mol and tryptophan -3.87 kcal/mol. ADMET prediction analysis showed that vindoline and catharanthine had promising pharmacokinetic and toxicity profiles, such as good blood-brain barrier (BBB) permeability and did not inhibit the CYP3A4 enzyme. These results indicate that vindoline and catharanthine are potential alternative leukemia treatments with high efficacy and low risk of side effects. This study provides a basis for further exploration of Catharanthus roseus in the development of effective and safe leukemia therapies.
References
Chennamadhavuni, A., Lyengar, V., Mukkamalla, S. K. R., & Shimanovsky, A. (2023). Leukemia. StatPearls. Treasure Island, FL: StatPearls Publishing.
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., & Jemal, A. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 74(3), 229-263.
Tebbi, C. K. (2021). Etiology of acute leukemia: A review. Cancers, 13(9), 2256.
Crossnohere, N. L., Richardson, D. R., Reinhart, C., O’Donoghue, B., Love, S. M., Smith, B. D., & Bridges, J. F. (2019). Side effects from acute myeloid leukemia treatment: results from a national survey. Current medical research and opinion, 35(11), 1965-1970.
Dasgupta, Y., Koptyra, M., Hoser, G., Kantekure, K., Roy, D., Gornicka, B., & Skorski, T. (2016). Normal ABL1 is a tumor suppressor and therapeutic target in human and mouse leukemias expressing oncogenic ABL1 kinases. Blood, The Journal of the American Society of Hematology, 127(17), 2131-2143.
Kim, J. C., Chan-Seng-Yue, M., Ge, S., Zeng, A. G., Ng, K., Gan, O. I., & Notta, F. (2023). Transcriptomic classes of BCR-ABL1 lymphoblastic leukemia. Nature genetics, 55(7), 1186-1197.
O'Hare, T., Shakespeare, W. C., Zhu, X., Eide, C. A., Rivera, V. M., Wang, F., & Clackson, T. (2009). AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer cell, 16(5), 401-412.
Aisyi, M., Lestari, P., Nadliroh, S., Meisita, A., Solachudin, D., Kristanti, D., & Karsono, B. (2020). The Profile of BCR-ABL1 Fusion Gene in Childhood Leukemia at “Dharmais” Cancer Hospital. Indonesian Journal of Cancer, 14(3), 86-90.
Almagro, L., Fernández-Pérez, F., & Pedreño, M. A. (2015). Indole alkaloids from Catharanthus roseus: bioproduction and their effect on human health. Molecules, 20(2), 2973-3000.
Zweil, A. M., Ibrahim, I. A., & Bekhit, M. (2019). Screening of Available Components of Egyptian Catharanthus Roseus Cultivars using HPLC-MS. Journal of Chemical and Pharmaceutical Research, 11(6), 33-40.
Sathya Prabhu, D., & Devi Rajeswari, V. (2017). Catharanthus roseus: The cancer-fighting medicine. Catharanthus roseus: Current Research and Future Prospects, 121-151.
Rajashekara, S., Reena, D., Mainavi, M. V., Sandhya, L. S., & Baro, U. (2022). Biological isolation and characterization of Catharanthus roseus (L.) G. Don methanolic leaves extracts and their assessment for antimicrobial, cytotoxic, and apoptotic activities. BMC Complementary Medicine and Therapies, 22(1), 328.
Lestariningrum, W. T., Kintoko, K., & Farid, M. (2024). Integrated Ethnomedicine Study in Silico of Medicinal Plants for Hypertension. Journal La Lifesci, 5(5), 483-501.
Dyas, R. A. A., & Wijianto, B. (2023). Docking studies for screening antibacterial compounds of Red Jeringau (Acorus calamus L.) using Shigella flexneri protein as a model system. Acta Chimica Asiana, 6(2), 343-350.
Pemovska, T., Johnson, E., Kontro, M., Repasky, G. A., Chen, J., Wells, P., et al. (2015, March 5). Axitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformation. Nature, 519(7541), 102–105.
Buannata, J., Wijianto, B., & Arief, I. (2024). Molecular docking study of modified isoniazid compounds on mycolic acid synthase in the cell wall of mycobacterium tuberculosis. Acta Chimica Asiana, 7(2), 471-477.
Bingöl Özakpinar, Ö., Türe, A., & Küçükgüzel, İ. (2020). Molecular modeling and assessment of cytotoxic and apoptotic potentials of imatinib analogues featuring (thio)urea motifs in human leukemia and lymphoma cells. Journal of Research in Pharmacy, 24(6), 801–811.
Reddy, E. P., & Aggarwal, A. K. (2012, May 1). The ins and outs of Bcr-Abl inhibition. Genes & Cancer, 3(5–6), 447–454.
Quintás-Cardama, A., & Cortes, J. (2009). Molecular biology of bcr-abl1–positive chronic myeloid leukemia. Blood, The Journal of the American Society of Hematology, 113(8), 1619-1630.
Pemovska, T., Johnson, E., Kontro, M., Repasky, G. A., Chen, J., Wells, P., et al. (2015, March 5). Axitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformation. Nature, 519(7541), 102–105.
Syahputra, R., Utami, D., & Widyaningsih, W. (2022). Studi docking molekuler aktivitas penghambatan enzim tirosinase ubi jalar (Ipomoea batatas L. Lam). Pharmacon: Jurnal Farmasi Indonesia, 19(1).
Bingöl Özakpinar, Ö., Türe, A., & Küçükgüzel, İ. (2020). Molecular modeling and assessment of cytotoxic and apoptotic potentials of imatinib analogues featuring (thio)urea motifs in human leukemia and lymphoma cells. Journal of Research in Pharmacy, 24(6), 801–811.
Reddy, E. P., & Aggarwal, A. K. (2012, May 1). The ins and outs of Bcr-Abl inhibition. Genes & Cancer, 3(5–6), 447–454.
Rossari, F., Minutolo, F., & Orciuolo, E. (2018). Past, present, and future of Bcr-Abl inhibitors: From chemical development to clinical efficacy. Journal of Hematology and Oncology, 11.
Xiong, B., Fan, M., Wang, Z., Yang, X., Cao, S., Shen, J., et al. (2024, November 1). Gentiopicroside-induced gastric cancer necroptosis via the HIF-1 signaling pathway: A study involving molecular docking and experimental validation. PLoS One, 19(11 November).
Sherin, D. R., & Manojkumar, T. K. (2021, December 1). Exploring the selectivity of guanine scaffold in anticancer drug development by computational repurposing approach. Scientific Reports, 11(1).
Liu, Z., Huang, L., Zhou, T., Chang, X., Yang, Y., Shi, Y., et al. (2022, July 1). A novel tubulin inhibitor, 6h, suppresses tumor-associated angiogenesis and shows potent antitumor activity against non–small cell lung cancers. Journal of Biological Chemistry, 298(7).
Dhyani, P., Quispe, C., Sharma, E., Bahukhandi, A., Sati, P., Attri, D. C., et al. (2022). Anticancer potential of alkaloids: A key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell International, 22.
Keglevich, A., Dányi, L., Rieder, A., Horváth, D., Szigetvári, Á., Dékány, M., et al. (2020, February 24). Synthesis and cytotoxic activity of new vindoline derivatives coupled to natural and synthetic pharmacophores. Molecules, 25(4).
Taher, Z. M., Agouillal, F., Lim, J. R., Marof, A. Q., Dailin, D. J., Nurjayadi, M., et al. (2019). Anticancer molecules from Catharanthus roseus. Indonesian Journal of Pharmacy, 30, 147–156.
Ahmad, H., Rahim, A., & Mat, I. (2010). Catharanthus roseus aqueous extract is cytotoxic to Jurkat leukaemic T-cells but induces the proliferation of normal peripheral blood mononuclear cells. Tropical Life Sciences Research, 21.
Wang, X. D., Li, C. Y., Jiang, M. M., Li, D., Wen, P., Song, X., et al. (2016, June 1). Induction of apoptosis in human leukemia cells through an intrinsic pathway by cathachunine, a unique alkaloid isolated from Catharanthus roseus. Phytomedicine, 23(6), 641–653.
Bates, D. J. P., Danilov, A. V., Lowrey, C. H., & Eastman, A. (2013, August). Vinblastine rapidly induces NOXA and acutely sensitizes primary chronic lymphocytic leukemia cells to ABT-737. Molecular Cancer Therapeutics, 12(8), 1504–1514.
Abdurrahman, S., Ruslin, R., Hasanah, A., & Mustarichie, R. (2021, April 1). Molecular docking studies and ADME-Tox prediction of phytocompounds from Merremia peltata as a potential anti-alopecia treatment. Journal of Advanced Pharmaceutical Technology & Research, 12(2), 132–139.
Daina, A., & Zoete, V. (2016). A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem, 11, 1117–1121.
Ononamadu, C. J., & Ibrahim, A. (2021). Molecular docking and prediction of ADME/drug-likeness properties of potentially active antidiabetic compounds isolated from aqueous-methanol extracts of Gymnema sylvestre and Combretum micranthum. Biotechnologia, 102(1), 85–99.
Sertel, S., Fu, Y., Zu, Y., Rebacz, B., Konkimalla, B., Plinkert, P. K., et al. (2011, March 15). Molecular docking and pharmacogenomics of Vinca alkaloids and their monomeric precursors, vindoline and catharanthine. Biochemical Pharmacology, 81(6), 723–735.
Rizkita, L. D., Putri, R. G. P., Farid, M., Rizkawati, M., & Wikaningtyas, P. (2024). Liposome drug delivery in combating the widespread topical antibiotic resistance: a narrative review. Beni-Suef University Journal of Basic and Applied Sciences, 13(1), 90.
License
Copyright (c) 2025 Muhammad Farid, Shalahuddin Al Madury, Ahmad Suriyadi Muslim, Zakiyyah Qurrotul ‘Aini

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish with ACA: Acta Chimica Asiana agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. This license allows authors to use all articles, data sets, graphics, and appendices in data mining applications, search engines, web sites, blogs, and other platforms by providing an appropriate reference. The journal allows the author(s) to hold the copyright without restrictions and will retain publishing rights without restrictions.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in ACA: Acta Chimica Asiana.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).





 Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia
Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia





