Binding studies of ruthenium complexes with antithyroid drug
Authors
D S Berlin Dinosha , George Allen Gnana Raj , Thangadurai Sumitha CelinDOI:
10.29303/aca.v7i2.213Published:
2024-10-31Issue:
Vol. 7 No. 2 (2024)Keywords:
hormones, drug, antithyroid, Benesi-Hildebrand plot, thyronorm, emissionArticles
Downloads
How to Cite
Downloads
Metrics
Abstract
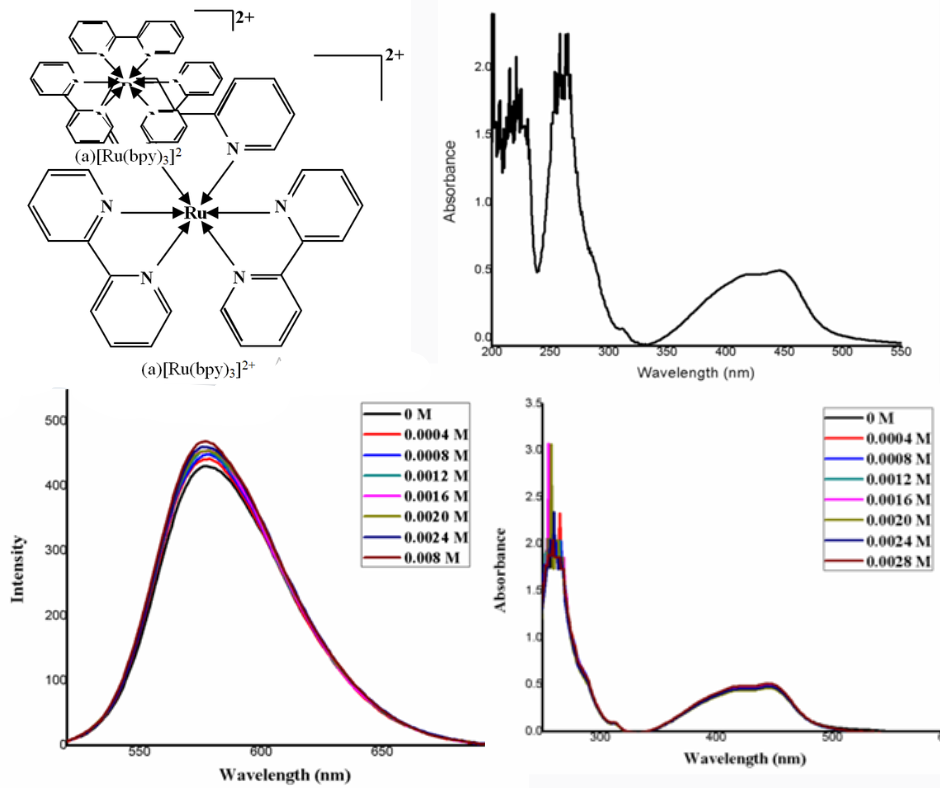
Medicinal substances are essential to our daily existence. A prudent approach to metal complexation with already available medications may result in more stylish, intelligent, and effective drug compositions. Thus, the binding of [Ru(NN)3]2+ (NN = 2,2ˈ-bipyridine, 1,10-phenanthroline) with the antithyroid medication thyronorm has been studied in the current attempt. The use of absorption and emission spectrum techniques allowed researchers to examine the complexes' interactions with antithyroid medications. Studies of absorption and emission spectra show that the complex and medication interact through coordinated ligand hydrophobic and hydrogen bonding interactions.Analysis has also been done on the binding of the antithyroid medication Thyronorm to ruthenium complexes. The Benesi-Hildebrand plot was used to determine the binding constants of the drug complexes. Current research indicates that the Thyronorm pill has good affinity with [Ru(phen)3]2+complex.
References
Soares L,A., Sardi J., Gullo F,P., Pitangui N,S., Scorzoni, L., Leite FS, et al.(2013). Anti dermatophytic therapy - prospects for the discovery of new drugs from natural products. Braz J Microbiol, 44,1035–41.
Islahudin, F, Tindall,S,M., Mellor, I,R., Swift, K., Christensen HEM, Fone KCF, et al(2014). The antimalarial drug quinine interferes with serotonin biosynthesis and action. Sci Rep [Internet]. 4(1):3618.
Maury, P., Rollin, A., Galinier, M., Juillière,Y.,(2014). Role of digoxin in controlling the ventricular rate during a trial fibrillation: a systematic review and a rethinking. Research Reports in Clinical Cardiology. 5,93–101.
Chraïbi, A., Renauld,S.,(2014).PPARγ-induced stimulation of amiloride-sensitive sodium current in renal collecting duct principal cells is serum and insulin dependent. Cell PhysiolBiochem. 33(3),581–93.
Grazul, M., Budzisz.E.,(2009). Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord Chem Rev.253(21–22),2588–98.
Ni, Y., Du, S., Kokot,S.,(2007). Interaction between quercetin-copper (II) complex and DNA with the use of the Neutral Red dye fluorophor probe. Analytica Chimica Acta. 584,19–27.
Etman, M.A., Salama,R,O., Shamsedeen, M,A., El-Kamel, A.,(2001). Solubilization of etodolac for parenteral administration. Indian J Pharm Sci. 63,459–67.
Li, P., Zhao L., Yalkowsky, S,H.,(1999). Combined effect of co solvent and cyclodextrin on solubilization of nonpolar drugs. J Pharm Sci.88,967–9.
Nielsen, A,B., Frydenvang, K., Liljefors, T., Buur, A., Larsen, C.,(2005). Assessment of the combined approach of N-alkylation and salt formation to enhance aqueous solubility of tertiary amines using bupivacaine as a model drug. Eur J Pharm Sci.24(1),85–93.
Otsuka, M., Matsuda,Y.,(1995) Effect of cogrinding with various kinds of surfactants on the dissolution behaviour of phenytoin. JPharm Sci. 84,1434–7.
Kumar,N,K., Murali, M., Prasad, C.,(2002).Himasankar K, Murthy VR. Comparative studies on the effect of some hydrophilic polymers on the dissolution rate of a poorly water-soluble drug meloxicam. Indian Drugs. 39(6),323–9.
Corvi,M,P., Cirri, M., Uekama, A,B., Fujinaga, K., Hirayama, T., Otagiri, F., et al.(1984). Enhancement of solubility and bioavailability by ternary complexation with β-cyclodextrin and glycine. J Pharm Sci. 92,1338–41.
Uekama, K., Fujinaga, T., Hirayama, F., Otagiri, M., Yamasaki, M., Seo, H., et al.(1983). Improvement of the oral bioavailability of digitalis glycosides by cyclodextrin complexation. J Pharm Sci.72(11),1338–41.
Duan, L., Mandal,F,B., Stewart, S., Privalov, B., Liobet, T., Sun, A., et al.(2011). A molecular ruthenium catalyst with water - oxidation activity comparable to that of photosystem II. Chemical Communications. 4,12607–9.
Chen, Z., Chen, C., Weinberg,D,R., Kang, P., Concepcion, J,J., Harrison, D,P., et al. (2011). Electrocatalytic reduction of CO2 to CO by polypyridyl ruthenium complexes. Chem Commun (Camb),47(47),12607–9.
Ohzu, S., Ishizuko, T., Hirai, Y., Fukuzum, S., Kojima,T.,(2013). Photocatalytic oxidation of organic compounds in water by using Ruthenium polypyridylamine complexes as catalysts with high efficiency and selectivity. An European Journal. 19,1563–7.
Muthu Mareeswaran, P., Babu, E., Rajagopal,S.,(2013). Optical recognition of anions by ruthenium (II) bipyridine - calyx [4] arene system. Journal of Fluorescence. 23,997–1006.
Babu, E., Muthu Mareeswaran, P., Rajagopal,S.,(2013). Highly sensitive optical biosensor for thrombin based on structure switching aptamer luminescent silica nanoparticles. Journal of Fluorescence. 23,137–46.
Muthu Mareeswaran, P., Maheshwara, D., Balu, E., Rajagopal,S.,(2012). Binding and Fluorescence, resonance energy transfer (FRET) of Ruthenium(II)bipyridine - calixarene system with proteins - experimental and docking studies. Journal of Fluorescence. 22,1345–56.
Taheri, S., Behzad, M., Nazari, H., Khaleghian,A.,(2013). Synthesis, characterization and biological studies of new Ruthenium polypyridyl complexes containing non innocent ligands. ISRN Inorganic Chemistry. 2013,1–6.
Gill,M,R., Thomas, J,R.,(2012). Ruthenium (II) Polypyridyl complexes and DNA - from structural probes to cellular imaging and therapeutics. Chemical Society Reviews. 41,3179–92.
Zhennan Zhao, Xiang Zhang , Chang-e Li , Tianfeng Chen(2019). Designing luminescent ruthenium prodrug for precise cancer therapy and rapid clinical diagnosis.Biomaterials.192,579-589.
Leipoldt,J,G., Lamprecht, G,J., Steynberg, E,C., (1991). Kinetics of the substitution of Acetylacetone in acetylactonate-1,5- cyclooctadiene rhodium (I) by derivatives of 1,10-Phenanthroline and 2,2’-dipyridyl. Journal of organometallic Chemistry. 402(2).
Sumitha Celin, T, Gnana Raj,A.,(2017), Micellar Effect on the Photoinduced Electron Transfer Reactions of Ruthenium (II)-Polypyridyl Complexes with Quinones. Journal of Chemical and Material Research. 6(2,3),1294–300.
Saha, B., Stanbury,D,M.,(2000). Thermal and Photochemical Reduction of Aqueous Chlorine by Ruthenium (II) Polypyridyl Complexes. Inorganic Chemistry.39,1294–300.
Author Biographies
D S Berlin Dinosha, Scott Christian College(Autonomous), Nagercoil, Tamilnadu, India
student
George Allen Gnana Raj, Scott Christian College(Autonomous), Nagercoil, Tamilnadu, India
Associate Professor of Chemistry
License
Copyright (c) 2024 D S Berlin Dinosha, George Allen Gnana Raj, Thangadurai Sumitha Celin

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish with ACA: Acta Chimica Asiana agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. This license allows authors to use all articles, data sets, graphics, and appendices in data mining applications, search engines, web sites, blogs, and other platforms by providing an appropriate reference. The journal allows the author(s) to hold the copyright without restrictions and will retain publishing rights without restrictions.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in ACA: Acta Chimica Asiana.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).





 Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia
Indonesian Chemical Society, Chapter Nusa Tenggara. Jalan Majapahit 62 Mataram, University of Mataram, 83125, Indonesia





